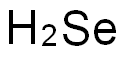Chemical Properties
Jewelers most frequently encounter selenium in the form of brass-black and gun-bluing
compounds. Selenium print toner used by photographers is sometimes used by jewelers
as a metal-coloring solution. These coloring mixtures usually contain selenic acid. Selenic
acid can release hydrogen selenide gas that can cause illness, and used daily, it might
enlarge the liver and spleen. Tellurium is sometimes used in association with selenium.
General Description
Selenium is a reddish colored powder that may become black upon exposure to air. SELENIUM POWDER(7782-49-2) is toxic by ingestion. SELENIUM POWDER(7782-49-2) is used to manufacture electronic components and rubber.
Reactivity Profile
SELENIUM, silicon, or sulfur ignites in fluorine gas at ordinary temperatures [Mellor 2:11-13 1946-47]. A mixture of barium carbide and selenium heated to 150° C becomes incandescence [Mellor 5:862 1946-47]. Calcium carbide and selenium vapor react with incandescence [Mellor 5:862 1946-47]. A moist mixture of selenium and chlorates, except the alkali chlorates, becomes incandescent. Selenium reacts violently with chromium trioxide [Mellor 11:233 1946-47]. Reaction of selenium and silver bromate (also potassium bromate) is violently explosive [Mellor 2, Supp1:763 1956]. Freshly reduced selenium reacts vigorously with nitric acid. Trace amounts of organic matter probably influenced the reaction [J. Chem. Soc. 1938 p.391]. The reaction between zinc and selenium or tellurium is accompanied by incandescence [Mellor 4:476-480 1946-47].
Air & Water Reactions
Insoluble in water.
Health Hazard
Exposures to selenium cause adverse health effects. selenium dioxide is a by-product of
copper and nickel melting, or of heating alloys containing selenium. The dusts are very
irritating to the mucous membranes and the lungs. Contact can cause dermatitis.
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Potential Exposure
Most of the selenium produced is used
in the manufacture of selenium rectifiers. It is also utilized
as a pigment for ruby glass, paints, and dyes; as a vulcaniz-
ing agent for rubber; a decolorizing agent for green glass; a
chemical catalyst in the Kjeldahl test; as an insecticide; in
the manufacture of electrodes, selenium photocells, sele-
nium cells, and semiconductor fusion mixtures; in photo-
graphic toning bathes; and for dehydrogenation of organic
compounds. It is also used in veterinary medicine and in
antidandruff shampoos. Se is used in radioactive scanning
for the pancreas and for photostatic and X-ray xerography.
It may be alloyed with stainless steel; copper, and cast
steel. Selenium is a contaminant in most sulfide ores of
copper, gold, nickel, and silver; and exposure may occur
while removing selenium from these ores.
Fire Hazard
Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, includ-
ing resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medi-
cal attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
Medical observation is recommended for 24 to 48 hours
after breathing overexposure, as pulmonary edema may be
delayed. As first aid for pneumonitis or pulmonary edema,
a doctor or authorized paramedic may consider administer-
ing a drug or other inhalation therapy.
Shipping
UN3283 Selenium compound, solid, n.o.s.,
Hazard Class: 6.1; Labels: 6.1-Poisonous material,
Technical Name Required.
Incompatibilities
Reacts violently with strong acids and
strong oxidizers, chromium trioxide; potassium bromate;cadmium. Reacts with incandescence on gentle heating
with phosphorous and metals, such as nickel, zinc, sodium,
potassium, platinum. Reacts with water @ 50 ? C forming
flammable hydrogen and selenious acids.
Description
Selenium was discovered in 1817 by J?ns Jacob Berzelius.
Especially noted was the similarity of the new element to the
previously known tellurium. Selenium is an essential trace
element atw0.1 ppm in diets. Selenium is a biologically active
part of a number of important proteins, particularly enzymes
involved in antioxidant defense mechanisms, thyroid hormone
metabolism, and redox control of intracellular reactions. In
humans and animals, selenium plays a role in protecting
tissues from oxidative damage as a component of glutathione
peroxidase.
Waste Disposal
Powdered selenium: dispose
in a chemical waste landfill. When possible, recover
selenium and return to suppliers
Isotopes
There are a total of 35 isotopes of selenium. Five of these are stable, anda sixth isotope has such a long half-life that it is also considered stable: Se-82 =0.83×10+20 years. This sixth isotope constitutes 8.73% of selenium’s abundance in theEarth’s crust, and the other five stable isotopes make up the rest of selenium’s abundanceon Earth.
Origin of Name
Named for the Greek word selene, meaning “moon.” Jons Jacob Berzelius
(1779–1848) discovered selenium and named it after the mineral called “eucairite,”
which in Greek means “just in time.”
Occurrence
Selenium is the 67th most abundant element in Earth’s crust. It is widely spread over theEarth, but does not exist in large quantities. As a free element it is often found with the elementsulfur.
There is only one mineral ore that contains selenium: eucairite (CuAgSe). Although rich inselenium, it is too scarce to be of commercial use. Almost all selenium is recovered from theprocessing of copper and the manufacturing of sulfuric acid as a leftover sludge by-product.This makes selenium’s recovery profitable. Recovering it from eucairite is not profitable.
Selenium is found in Mexico, Bosnia, Japan, and Canada. It can be found in recoverablequantities in some soils in many countries.
Characteristics
Crystalline selenium is a p-type semiconductor. It acts as a rectifier that can change electriccurrent from alternating current (AC) to direct current (to DC). It has photovoltaic proper ties, meaning it is able to convert light (radiant) energy that strikes it into electrical energy.Selenium’s resistance to the flow of electricity is influenced by the amount of light shining onit. The brighter the light, the better the electrical conductivity.
Selenium burns with a blue flame that produces selenium dioxide (SeO2). Selenium willreact with most metals as well as with nonmetals, including the elements in the halogen group17.
Definition
A metalloid element
existing in several allotropic forms
and belonging to group 16 of the periodic
table. It occurs in minute quantities in sulfide
ores and industrial sludges. The common
gray metallic allotrope is very
light-sensitive and is used in photocells,
solar cells, some glasses, and in xerography.
The red allotrope is unstable and reverts
to the gray form under normal
conditions.
Symbol: Se; m.p. 217°C (gray); b.p.
684.9°C (gray); r.d. 4.79 (gray); p.n. 34;
r.a.m. 78.96.
Definition
selenium: Symbol Se. A metalloidelement belonging to group 16 (formerlyVIB) of the periodic table; a.n.34; r.a.m. 78.96; r.d. 4.81 (grey); m.p.217°C (grey); b.p. 684.9°C. There are anumber of allotropic forms, includinggrey, red, and black selenium. Itoccurs in sulphide ores of other metalsand is obtained as a by-product(e.g. from the anode sludge in electrolyticrefining). The element is asemiconductor; the grey allotrope islight-sensitive and is used in photocells,xerography, and similar applications.Chemically, it resemblessulphur, and forms compounds withselenium in the +2, +4, and +6 oxidation states. Selenium was discoveredin 1817 by J?ns Berzelius.
Production Methods
Selenium (Se), a nonmetallic element of the sulfur group, is
widely distributed in nature. It is obtained along with tellurium
as a by-product of metal ore refining, chiefly from
copper deposits. About 16 ton is mined a year globally.
The global refinery production of selenium, excluding the
U.S. production, increased from about 1,400 metric ton in
2000 to about 1510 metric ton in 2008 and 1500
in 2009.
Because selenium is present in fossil fuels, up to 90% of
the selenium content in ambient air is emitted during their
combustion. Air pollution concentrations averaged from
0.38 ng/m3 in remote areas to 13 ng/m3 in urban areas.
The mass medium particle diameter was 0.92 mm. The
worldwide emissions of 10,000 tons/year from natural
sources exceed the atmospheric emissions from anthropogenic
sources (5100 ton). However, 41,000 tons is emitted
into the aquatic ecosystems. The largest contributors are
electric power generating plants that produce 18,000 ton;
manufacturing processes account for 12,000 ton.
Most of the world’s selenium today is provided by
recovery from anode muds of electrolytic copper
refineries. Selenium is recovered by roasting these muds
with soda or sulfuric acid or by melting them with a soda
and niter.
Hazard
The fumes and gases of most selenium compounds are very toxic when inhaled. SeO2 andSeS2 are toxic if ingested and very irritating to the skin. They are also carcinogenic.
Although some compounds of selenium are poisonous, as an element it is essential in traceamounts for humans. It is recommended that 1.1 to 5 milligrams of selenium be included inthe daily diet. This amount can be maintained by eating seafood, egg yokes, chicken, milk,and whole grain cereals. Selenium assists vitamin E in preventing the breakdown of cells andsome chemicals in the human body.
Flammability and Explosibility
Nonflammable
Agricultural Uses
Selenium (Se) is a metalloid element belonging to Group
16 (formerly VIB) of the Periodic Table. It is an
essential ingredient in the forage for animals to prevent
muscular dystrophy or white muscle disease which
weakens the heart of cattle and sheep.
However, selenium is not essential for plants, and its
uptake by plants varies. Certain species of Astrugals
absorb more selenium than others because of a special
amino acid in them. Plants like mustard, cabbage and
onions absorb moderate amounts of selenium. This
absorbed selenium accumulates in the tissues of these
plants, and no treatment can remove it. The excess soil
selenium content can be corrected by the addition of
barium chloride or calcium sulphate, which may form
insoluble selenate.
Chemically, selenium resembles sulphur. Its total
concentration in most soils is between 0.1 and 0.3 ppm as
selenides, elemental selenium, selenites, selenates and
organic selenium compounds. The selenium uptake is the
highest in basic soil and the lowest in neutral soil.
There has been some concern about the increased
selenium deficiencies in cattle due to a negative effect of
sulphate on the selenate ion uptake by crops. Such
livestock disorders are severe after a wet summer. This is
due to a lowered soil redox potential, converting selinium
into forms unavailable for plant uptake. This is also
pronounced in soils with increased nitrate deposition
which converts the selenate and selenite into elemental
selenium or its gaseous form. On the other hand, winter
forage is seen to contain higher amounts of selenium.
Phosphate rocks and superphosphates containing 20
ppm or more of selenium may be sufficient for plants to
protect the livestock from being deficient in selenium.
Fertilization programs to produce selenium-adequate
forage, specifically suited to grazing animals, are a
subject of continuing interest. Fertilization with selenites
is preferred to other easily available selenates in view of
the former's slow-acting nature. Fertilization with
selenites is preferred also because they produce a lesser
level of selenium in plants than selenates do. Selenium of
roughly 75 g/ha for forages and 15 g/ha for foliar
application is satisfactory.
Biotechnological Applications
Selenium is a component of a number of proteins. Selenium can exist as an anion at biological pH, which makes it able to both give and accept electrons. The best understood physiological functions of selenium are two enzyme functions. One of these functions is done as part of a family of proteins named glutathione peroxidase (one is found inside of cells, another is outside cells in places like the plasma).
Glutathione peroxidase is part of the body's antioxidant defense network by eliminating peroxides, including hydrogen peroxide, which can be both precursors and products of free radicals. Selenium also functions in an enzyme that is part thyroid hormone synthesis. A more recently discovered selenium enzyme is known as thioredoxin reductase, which seems to have a number of regulatory roles within cells, and seems to affect antioxidant defense by inßuencing electron ßow in some reactions. One interesting point about this enzyme is that in rats, the enzyme activities can be increased by elevating selenium intake above those normally considered adequate.
Veterinary Drugs and Treatments
Depending on the actual product and species, vitamin E/selenium
is indicated for the treatment or prophylaxis of selenium-tocopherol
deficiency (STD) syndromes in ewes and lambs (white muscle
disease), sows, weanling and baby pigs (hepatic necrosis, mulberry
heart disease, white muscle disease), calves and breeding cows
(white muscle disease), and horses (myositis associated with STD).
Vitamin E may be useful as adjunctive treatment of discoid lupus
erythematosus, canine demodicosis, and acanthosis nigricans in dogs. It may also be of benefit in the adjunctive treatment of hepatic
fibrosis or adjunctive therapy of copper-associated hepatopathy
in dogs.
Environmental Fate
Although selenium occurs naturally in the environment found
in rocks and soil, it can also be released by both natural and
manufacturing processes. However, forms of selenium can be
transformed (changed) in the environment. Weathering of
rocks to soil may cause low levels of selenium in water or it may
cause it to be taken up by plants and naturally released into the
air. Volcanic eruptions are suspected of contributing to selenium
in air, and soils in the areas around volcanoes tend to
have enriched amounts of selenium.
Selenium has multiple oxidation states (valence states)
including -2, 0, +4, and +6. The type of selenium found is
a result of its oxidation state, which may vary according to
ambient conditions, such as pH and microbial activity. Selenium
enters the air from burning coal or oil. Most of the selenium in
air is bound to fly ash and to suspended particles. The elemental
selenium that may be present in fossil fuels forms selenium
dioxide during combustion (burning). Selenium dioxide can
then form selenious acid with water or sweat. Selenium anhydride is released during the heating of copper, lead, and zinc
ores when there is selenium in them. Hydrogen selenide
decomposes rapidly in air to form elemental selenium and
water, thus eliminating the danger from this compound formost
people, except those who are exposed to it in their workplace.
Airborne particles of selenium, such as in coal ash, can settle
on soil or surface water. Disposal of selenium in commercial
products and waste could also contribute to selenium levels in
soil. But the amount of selenium released to soil from fly ash
and hazardous waste sites has not been measured. The forms
and fate of selenium in soil depend largely on the acidity of the
surroundings and its interaction with oxygen. In theory,
at equilibrium with no oxygen present, deep-soil selenium may
be present as elemental selenium. In the absence of oxygen
when the soil is acidic, the amount of biologically available
selenium should be low. Elemental selenium that cannot
dissolve in water and other insoluble forms of selenium (such
as selenium sulfide and heavy metal selenides) are less mobile
and will usually remain in the soil, posing less of a risk for
exposure. Active agricultural or industrial processes may
increase the amount of biologically available selenium by
decreasing the acidity of the soil and increasing the oxygen and
the soluble selenium compounds. Selenium compounds that
can dissolve in water are very mobile. For example, selenates
and selenites are water-soluble, and thus mobile, so there is an
increased chance of exposure to them. Irrigation drainage
waters may result in increased selenium entering the surface
water. Other factors that may affect the rates at which selenium
moves through the soil are temperature, moisture, time, season
of year, concentration of water-soluble selenium, organic
matter content, and microbiological activity.
Purification Methods
Dissolve selenium in small portions in hot conc HNO3 (2mL/g), filter and evaporate to dryness to give selenious acid which is then dissolved in conc HCl. Pass SO2 gas through the solution whereby selenium (but not tellurium) precipitates. It is filtered off and washed with conc HCl. This purification process is repeated. The selenium is then converted twice to the selenocyanate by treating with a 10% excess of 3M aqueous KCN (CARE), heated for half an hour on a sand-bath and filtered. Add an equal weight of crushed ice to the cold solution, followed by an excess of cold, conc HCl, with stirring (in an efficient fume cupboard as HCN is evolved) which precipitates selenium powder. This is washed with water until colourless, and then with MeOH and is heated in an oven at 105o. Finally it is fused for 2hours in vacuo. It is cooled, crushed and stored in a desiccator [Tideswell & McCullough J Am Chem Soc 78 3036 1956].
Structure and conformation
It takes three types of structures, metallic (grey) selenium, crystal (red) selenium, and amorphous selenium. The space lattice of metallic selenium belongs to the hexagonal system with two types, A and B. The B type is the most stable and the quasi-stable; A type changes to the B type slowly. The structure of the B type is an infinite zigzag chain containing three atoms in a unit cell with lattice constant of a=0.4355 nm, c=0.4949 nm, Se–Se=0.232 nm, and <Se– Se–Se=105° . The space lattice of crystal belongs to the monoclinic system with a=0.905 nm, b=0.907 nm, c=1.161 nm, Se–Se=0.234 nm, b=90841' , and <Se–Se–Se=105.38° ±2.3° . There may be two types for crystal selenium. Amorphous selenium changes to metallic selenium slowly at room temperature. Sometimes, selenium is classified into trigonal and amorphous types3 .
Toxicity evaluation
Selenium in the body can be grouped in three main categories:
selenium in proteins, nonprotein selenium species, and selenoamino
acids. The most prevalent selenium species include
selenocysteine, selenomethionine, and inorganic forms of selenium
(selenite and selenate). Little is known about the specific
biochemical mechanisms by which selenium and selenium
compounds exert their acute toxic effects but may involve redox
cycling. Generally, water-soluble forms are more easily absorbed
and are generally of greater acute toxicity. Sulfhydryl enzymes are
attacked by soluble selenium compounds.
Excess selenium results in liver atrophy, necrosis, and
hemorrhage.