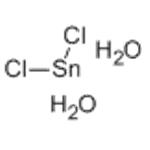
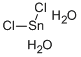Chemical properties
It appears as colorless or white monoclinic crystal, being soluble in alcohol, ether, acetone and glacial acetic acid.
Solubility in water
Solubility in 100 ml of water:84 g/0 ° C
Identification test
Use a dilute hydrochloric acid test solution (TS-117) to prepare a 5% sample solution. Add drop wise of the mercuric chloride test solution (TS-138) to produce a white or off-white precipitate.
The result of the chloride test (IT-12) of the 5% sample solution was positive.
Uses
(1) It can be used as a reducing agent, mordant, bleaching agent and for tin plating in the electroplating industry.
(2) Stannous chloride dihydrate can be used for the colorimetric determination of silver, lead, arsenic and molybdenum, being also be used as a reducing agent and mordant
(3) This product is mainly used in acidic tin plating as major salt. Tin is in bivalent in the bath with high efficiency cathode. The general usage amount is 40~60 kg/L. It can also be used for glass mirror industry, as silver nitrate sensitizer to enable excellent coating brightness. Addition of the plating layer of this product during the ABS electroplating is not easy to fall off.
(4) Stannous chloride dihydrate can be used as the reducing agents in the manufacture of dyestuff intermediates. It can act as the components of super high pressure lubricating oil. It can be used as a bleaching agent for the sensitization of the mercury-plating during the manufacturing of mirror, enables excellent brightness of the formed silver film so that the combination of mercury and products is quite firm. Electroplating industry applied it for the tin-plating, copper-tin plating of the mechanical parts. During the ABS plastic plating, it is used for sensitization so that the coating is not easy to fall off. It can also be used in the synthesis of pharmaceuticals, as the catalyst in organic synthesis and the activator of the butyl rubber vulcanization. It can also be used as the mordant and the anti-dyeing agent in printing discharge process. It can be used as the stabilizers for perfumery industry, as food reductant and antioxidants used for canned asparagus, pineapple juice.
(5) It can be used as analysis reagents and reducing agent. It can also be used as the reductant for the production of dye intermediates; it can also be used for electroplating; as brightening agent during galvanized silver plating and plastic plate plating; as perfume stabilizer, bleach, stabilizing soap aroma; oil anti-fouling agent; raw material for organic synthesis; the raw material of herbicide Oxadiazon.
(6) It is a kind of strong reducing agent. It can be used for the determination of hydride via AAS; colorimetric determination of silver, lead, arsenic and molybdenum; determination of serum inorganic phosphorus and alkaline phosphatase activity, molybdenum blue method for the determination of soil and plant phosphorus content; the catalyst of the organic reaction.
(7) As a strong reducing agent, it is used for carbonyl allylation reaction; as the Lewis acid catalyst in a C-C bond reaction; the catalyst for the co-acting with AgClO4 for the synthesis of α-glycosides, the synthesis of such olefins, diolefins, cis-vinyl ethylene oxide and allyl selenide and de-oxidation of internal peroxides; used for the protection of carboxylic acids in the presence of 1,3-dithianes and selective methoxybenzyl ether scavenging reagents; the additives in hydroformylation and carbonylation reactions.
Content analysis
Accurately weigh about 2 g of sample and place it into a 250 ml volumetric flask. Add 15 l hydrochloric acid to solve it and use water to se the final volume, mix uniformly. Take 50 mL of this solution to place in a 500ml flask, add 5g potassium sodium tartrate and mix uniformly. Apply the cold saturated solution of sodium bicarbonate to adjust to alkaline (with litmus paper) and immediately titrate with 0.1mol/L iodine solution and take starch test solution (TS-235) as the indicator. Every mL of 0.1 mol/L iodine solution corresponds to 9.48 mg of stannous chloride (SnCl2) or 11.28 mg of stannous chloride dihydrate (SnC12.2H2O).
Toxicity
ADI 0~2mg/kg (FAO/WHO, 2001); Irritating to skin; Toxic, allowable content of air: 2mg/m. (In terms of Sn);
GRAS (FDA, § 184.1845, 2000);
LD50: 700 mg/kg (rat, oral);
During the production process of making Tin flower, we should avoid inhalation of tin dust, so as to avoid suffering from chronic bronchitis. The contact of stannous chloride solution with the skin can cause eczema. The maximum allowable concentration of the inorganic compound of tin in the United States is 2 mg/m3 (calculated based on metal tin). The production personnel should wear overalls, wear protective masks and gloves and other labor insurance products, paying attention to the protection of respiratory organs, protect the skin. The production equipment should be closed and the workshop should be well ventilated.
Usage limit
FDA/WHO(1984, mg/kg): canned asparagus 25 (only used for glass bottles of painted cans of canned asparagus, calculated based on Sn); pineapple juice 8 that merely preserved by physical methods (for reconstituted fruit juices, fruit juice prepared from frozen concentrated juice).
FDA, §172.180 (2000): canned asparagus color protection, 20mg/kg (Sn).
FDA, § 184.1845 (2000): various foods, 0.0015% (in Sn).
Production method
Tin can be dissolved in hydrochloric acid to derive.
Hydrochloric acid method: first melt the metal tin, and then pour into cold water to enable to the formation of the tin flower. Hydrochloric acid and tin flower are further added at a certain proportion into the reactors for reaction until the solution concentration of 40 ° Bé or so. It is further put into the enamel evaporator for concentration. First of all, add tin flower to the evaporator; through steam heating, have hydrochloric acid to further react with tin. When the solution concentration is increased to 73~77 ° Bé, filter while hot and then cool for crystallization and perform centrifugal separation to obtain the finished product of Tin. its
Sn + 2HCl → SnC12 + H2 ↑
Chemical Properties
Stannous chloride is a white crystalline solid.
Chemical Properties
White solid
Physical properties
White orthogonal crystal; density 3.90 g/cm3; melts at 247°C; vaporizes at 623°C; vapor pressure 1 torr at 316°C, 5 torr at 366°C and 20 torr at 420°C; soluble in water, ethanol, acetone and ether; insoluble in xylene and mineral spirits.
Uses
Tin(II) chloride dehydrate may be used:
- As a reducing agent for the determination of hydride forming species by AAS.
- In the conversion of organomercurials into inorganic mercury determined using a flow injection-cold vapor atomic absorption spectrometry
- In the determination of total mercury content, using atomic fluorescence spectroscopy and mercury speciation was performed using gas chromatography inductively coupled plasma mass spectrometry (GC-ICPMS).
Uses
Stannous chloride is used as a sensitizing agent in preparing glass and plastic for metalizing. It serves as a potent reducing agent. Other uses of stannous chloride are tin electroplating baths, corrosion inhibitors, polymers, thermoplastic elastomers, soldering flux, antioxidant, tanning agent and pharmaceuticals.
Uses
Powerful reducing agent, particularly in manufacture of dyes and 99mTc radiopharmaceuticals; in tinning by galvanic methods; in liquor finishing of wire; in sensitizing of glass and plastics before metallizing; as soldering flux; as mordant in dyeing with cochineal; in manufacture of tin chemicals, color pigments, pharmaceuticals, sensitized paper, lubricating oil additives; as tanning agent; in removing ink stains; in yeast revivers; as reagent in analytical chemistry; as catalyst in organic reactions.
Definition
ChEBI: A hydrate that is tin dichloride (anh.) combined with 2 mol eq. of water.
General Description
Crystals of SnCl
2.2H
2O exhibit monoclinic crystal system and space group
P2
1/
c.
Potential Exposure
Stannous chloride is used as a dye, pigment, and printing ink; in making chemicals; chemical preservatives; food additives; polymers, textiles, glass, silvering mirrors.
Shipping
UN3260 Corrosive solid, acidic, inorganic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required.
Incompatibilities
A strong reducing agent. Reacts violently with oxidants. Reacts violently with bromine trifluoride; potassium, hydrazine hydrate, sodium, sodium peroxide; ethylene oxide; and nitrates. Keep away from moisture, sources of oxygen, and combustible materials.