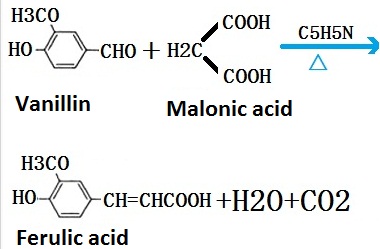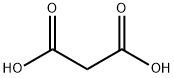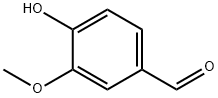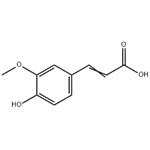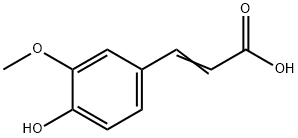
Ferulic Acid
- Product NameFerulic Acid
- CAS1135-24-6
- CBNumberCB0337151
- MFC10H10O4
- MW194.18
- EINECS214-490-0
- MDL NumberMFCD00004400
- MOL File1135-24-6.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 168-172 °C(lit.) |
| Boiling point | 250.62°C (rough estimate) |
| Density | 1.316(20.0000℃) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.5168 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | powder |
| pka | 4.58±0.10(Predicted) |
| color | slightly yellow |
| Water Solubility | soluble |
| InChIKey | KSEBMYQBYZTDHS-HWKANZROSA-N |
| LogP | 1.51 |
| CAS DataBase Reference | 1135-24-6(CAS DataBase Reference) |
| EWG's Food Scores | 1-2 |
| FDA UNII | AVM951ZWST |
| NIST Chemistry Reference | Cinnamic acid, 4-hydroxy-3-methoxy-(1135-24-6) |
| EPA Substance Registry System | Ferulic acid (1135-24-6) |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36-37/39 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | UD3365500 | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29162090 | |||||||||
| Hazardous Substances Data | 1135-24-6(Hazardous Substances Data) | |||||||||
| Toxicity | mouse,LD,intraperitoneal,> 350mg/kg (350mg/kg),BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY)BEHAVIORAL: ATAXIABEHAVIORAL: REGIDITY,Indian Journal of Pharmaceutical Sciences. Vol. 49, Pg. 77, 1987. | |||||||||
| NFPA 704: |
|