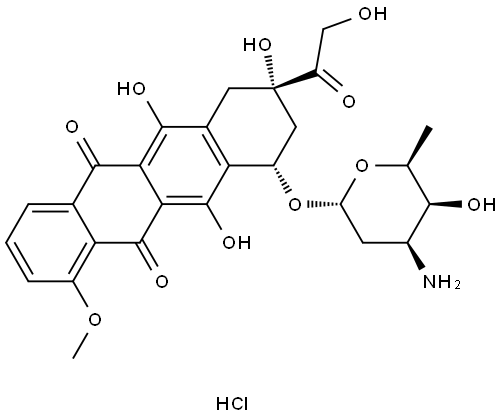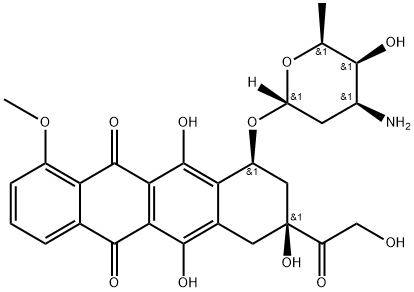
Adriamycin
- Product NameAdriamycin
- CAS23214-92-8
- CBNumberCB1170865
- MFC27H29NO11
- MW543.53
- EINECS245-495-6
- MDL NumberMFCD00869292
- MOL File23214-92-8.mol
Chemical Properties
| Melting point | 205°C |
| Boiling point | 617.77°C (rough estimate) |
| Density | 1.3783 (rough estimate) |
| refractive index | 1.6400 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | ≥27.2 mg/mL in DMSO; insoluble in EtOH; ≥24.8 mg/mL in H2O with ultrasonic |
| form | solid |
| pka | pKa 8.2 (Uncertain) |
| Water Solubility | Soluble |
| CAS DataBase Reference | 23214-92-8 |
| EWG's Food Scores | 5 |
| NCI Dictionary of Cancer Terms | Adriamycin PFS; Adriamycin RDF; Doxil |
| FDA UNII | 80168379AG |
| NCI Drug Dictionary | Doxil |
| ATC code | L01DB01 |
| IARC | 2A (Vol. 10, Sup 7) 1987 |
| EPA Substance Registry System | Doxorubicin (23214-92-8) |
Safety
| Symbol(GHS) |
 
|
| Signal word | Danger |
| Hazard statements | H302-H315-H319-H335-H350-H362 |
| Precautionary statements | P260-P263-P280 |
| HS Code | 2941900000 |
| Hazardous Substances Data | 23214-92-8(Hazardous Substances Data) |
| Toxicity | An anthracycline cytotoxic antineoplastic that is produced by Streptomyces peucetius. The LD50 in mice is 9.4 mg/kg, i.v. It is a carcinogen that inhibits DNA and RNA synthesis by intercalating in double-stranded DNA with the amino sugar in the minor groove and the 90-OH group of the anthracycline ring hydrogen-bonded to the adjacent guanine. It also alters membrane fluidity and ion transport, and generates free radicals through a cytochrome P450-mediated reductive process. In humans, it causes alopecia, stomatitis, nausea, vomiting, diarrhea, cardiotoxicity (manifested by tachycardia), and potentially fatal congestive heart failure. |