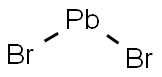Physical Properties
White orthorhombic crystals; density 6.66 g/cm3; melts at 373°C; forms a horn-like mass on solidification; vaporizes at 916°C; decomposes slowly on exposure to light; sparingly soluble in cold water (4.55 g/L at 0°C and 8.44 g/L at 20°C, respectively); moderately soluble in boiling water (44.1g/L at 100°C); Ksp 6.60x10–6 at 25°C; insoluble in alcohol; slightly soluble in ammonia; soluble in alkalies and also in sodium or potassium bromide solutions.
Uses
Lead(II) bromide is used for developing images in photography; as inorganic filler in fire-retardant plastics; as a photopolymerization catalyst for acrylamide monomer; and as a welding flux for welding aluminum or its alloys to other metals.
Preparation
Lead bromide is prepared by treating an aqueous solution of lead nitrate with hydrobromic acid or with sodium or potassium bromide:
Pb
2+ + 2Br¯ → PbBr2
The solution is allowed to stand to let the precipitate settle.
The compound also may be obtained by adding lead carbonate or lead monoxide to hydrobromic acid.
Toxicity
Moderately toxic by ingestion. The toxic effects are those of lead.
Chemical Properties
white, ortho-rhomb crystal(s); -80 mesh with 99.999% purity; enthalpy of vaporization 133 kJ/mol; enthalpy of fusion 16.44 kJ/mol; obtained from PbO or PbCO3 and HBr; finds use as a photopolymerization catalyst and in photoduplication processes in the 365 nm region [KIR78] [CER91] [CRC10] [MER06]
Physical properties
White orthorhombic crystals; density 6.66 g/cm
3; melts at 373°C; forms a horn-like mass on solidification; vaporizes at 916°C; decomposes slowly on exposure to light; sparingly soluble in cold water (4.55 g/L at 0°C and 8.44 g/L at 20°C, respectively); moderately soluble in boiling water (44.1g/L at 100°C); Ksp 6.60x10
-6 at 25°C; insoluble in alcohol; slightly soluble in ammonia; soluble in alkalies and also in sodium or potassium bromide solutions.
Uses
Lead bromide is used for developing images in photography; as inorganic filler in fire-retardant plastics; as a photopolymerization catalyst for acrylamide monomer; and as a welding flux for welding aluminum or its alloys to other metals.
Uses
Lead(II) bromide (PbBr
2) can be used in the fabrication of nanoscale quasi-2D layered perovskites, which are potentially utilized as light-emitting materials. It can also be used for the synthesis of deep blue fluorescent lead bromide perovskite microdisks. These microdisks can be used as direct bandgap semiconductors for light-emitting diodes (LEDs).
Uses
It is used in the field of antirust, pigment and photograph. The molten lead(II) bromide acts as an electrolyte. Lead(II) bromide provides a high concentration of lead(II) ions and bromide ions to carry the current during the electrolysis process. The rare-earth-doped alkali-lead bromide crystals (potassium lead bromide (or) KPB; rubidium lead bromide or RPB) emerge as promising new low-phonon-energy host materials for mid-IR applications and are useful for solid state lasers. Hybrid organic/lead halide perovskites are promising materials for solar cell fabrication.
Purification Methods
Crystallise it from water containing a few drops of HBr (25mL of water per gram PbBr2) between 100o and 0o. A neutral solution is evaporated at 110o, and the crystals that separate are collected by rapid filtration at 70o and dried at 105o (to give the monohydrate). Its solubility in H2O is 0.5% (at ~10o) and 5% (at ~ 100o). To prepare the anhydrous bromide, the hydrate is heated for several hours at 170o and then in a Pt boat at 200o in a stream of HBr and H2. Finally it is fused [Clayton et al. J Chem Soc, Faraday Trans 1 76 2362 1980].