Physical Properties
Bluish-green fine powder; hygroscopic. The monohydrate is dimeric; density 1.88 g/cm3; melts at 115°C; decomposes at 240°C; soluble in water and ethanol; and slightly soluble in ether.
Uses
Copper(II) acetate is used as a pigment for ceramics; in the manufacture of Paris green; in textile dyeing; as a fungicide; and as a catalyst.
Preparation
Copper(II) acetate is prepared by treatment of copper(II) oxide, CuO, or copper(II) carbonate, CuCO3, with acetic acid, followed by crystallization:
CuO + 2CH3COOH → (CH3COO)2Cu + H2O
Toxicity
Copper(II) acetate is moderately toxic by ingestion and possibly other routes of administration.
LD50 oral (rat): c. 600 mg/kg
Description
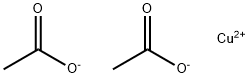
Copper (II) acetate, also referred to as cupric acetate, is the chemical compound with the formula Cu(OAc)2
2 where OAc
- is acetate (CH
3CO
2-). The hydrated derivative, which contains one molecule of water for each Cu atom, is available commercially. Anhydrous Cu(OAc)2 is a dark green crystalline solid, whereas Cu
2(OAc)
4(H
2O)
2 is more bluish-green. Since ancient times, copper acetates of some form have been used as fungicides and green pigments. Today, copper acetates are used as reagents for the synthesis of various inorganic and organic compounds. Copper acetate, like all copper compounds, emits a blue-green glow in a flame.
Chemical Properties
Cupric acetate is a greenish Blue powder or small crystals.
History
Copper (II) acetate was historically prepared in vineyards, since acetic acid is a byproduct of fermentation. Copper sheets were alternately layered with fermented grape skins and dregs left over from wine production and exposed to air. This would leave a blue substance on the outside of the sheet. This was then scraped off and dissolved in water. The resulting solid was used as a pigment, or combined with arsenic trioxide to form copper acetoarsenite, a powerful insecticide and fungicide called Paris Green or Schweinfurt Green.
During the Second World War copper acetate was used as shark repellent . Under war conditions, before adoption it has been tested only very briefly (while in general successfully). The source says copper acetate does repel sharks in some situations but not in all.
Uses
Used as a catalyst or oxidizing agent in organic syntheses
Uses
Copper(II) acetate is used in biochemical applications such as DNA extraction. It is used as a source of copper in inorganic synthesis, an oxidizing agent and catalyst in organic synthesis. It is also used to couple two terminal alkynes to make a 1,3-diyne. It is widely used in pigments, manufacture of paris green, textile dyeing, skin conditioner in cosmetics, antioxidant and stabilizer in food grade polymers.
Application
The uses for copper (II) acetate are more plentiful as a catalyst or oxidizing agent in organic syntheses. For example, Cu
2(OAc)
4 is used to couple two terminal alkynes to make a 1,3-diyne:
Cu
2(OAc)
4 + 2 RC ≡ CH → 2 CuOAc + RC ≡ C-C ≡ CR + 2 HOAc
The reaction proceeds via the intermediacy of copper(I) acetylides, which are then oxidized by the copper(II) acetate, releasing the acetylide radical. A related reaction involving copper acetylides is the synthesis of ynamines, terminal alkynes with amine groups using Cu
2(OAc)
4.
General Description
A blue-green crystalline solid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Cupric acetate is used as an insecticide, in the preparation of other chemicals, as a fungicide, and mildew preventive.
Air & Water Reactions
Water soluble.
Reactivity Profile
Salts, basic, such as Cupric acetate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Health Hazard
Inhalation of dust causes irritation of throat and lungs. Ingestion of large amounts causes violent vomiting and purging, intense pain, collapse, coma, convulsions, and paralysis. Contact with solutions irritates eyes; contact with solid causes severe eye surface injury and irritation of skin.
Fire Hazard
Special Hazards of Combustion Products: Irritating vapors of acetic acid may form in fires.
Safety Profile
Poison by
subcutaneous and intraperitoneal routes.
Moderately toxic by ingestion.
Experimental reproductive effects. When
heated to decomposition it emits acrid
smoke and irritating fumes. See also
COPPER COMPOUNDS.
Synthesis
Copper (II) acetate synthesized by the method described in the history section leads to impure samples. It is prepared industrially by heating copper (II) hydroxide or copper (II) carbonate with acetic acid. A second method of copper acetate production is to electrolyze a concentrated aqueous solution of calcium acetate with copper electrodes. As the reaction proceeds the anode oxidizes to produce copper acetate which adheres to its surface and can be removed as crystals. At the cathode calcium ions are reduced to calcium atoms and would be deposited, but due to the water content of the solution the calcium is converted to insoluble calcium hydroxide. The drawback with this setup is that the cathode gets coated with an insulating layer of calcium hydroxide, which gradually slows the process. To negate this hydroxide buildup mercury is utilized as the cathode; therefore as the process takes place the calcium formed immediately reacts with the mercury to make a calcium-mercury amalgam and the copper acetate formed at the anode is removed periodically. This process generally yields suitably pure copper acetate, on a small scale, with slight traces of calcium acetate.
Copper (II) acetate also forms by treating copper metal with a solution of acetic acid and hydrogen peroxide.
Potential Exposure
Cupric acetate is used as a fungicide, as a catalyst for organic reactions; in textile dyeing and as a pigment for ceramics.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heartactionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention.Give large quantities of water and induce vomiting. Do notmake an unconscious person vomit. Medical observation isrecommended for 24-48 h after breathing overexposure, as pulmonary edema may be delayed. As first aid for pulmonaryedema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
storage
Color Code—Green: General storage may be used.Prior to working with this chemical you should be trained on its proper handling and storage. Store in tightly closed containers in a cool, well-ventilated area. Cupric acetatemust be stored to avoid contact with acetylene gas, chemically active metals (such as potassium, sodium, magnesium,and zinc), since violent reactions occur
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Recystallise it twice from warm dilute acetic acid solutions (5mL/g) by cooling. [Beilstein 2 IV 111.]
Incompatibilities
Forms explosive materials with acetylene gas, ammonia, caustic solutions; sodium hypobromite; notromethane. Keep away from chemically active metals; strong acids; nitrates. Decomposes above 240C forming acetic acid fumes
Waste Disposal
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill.