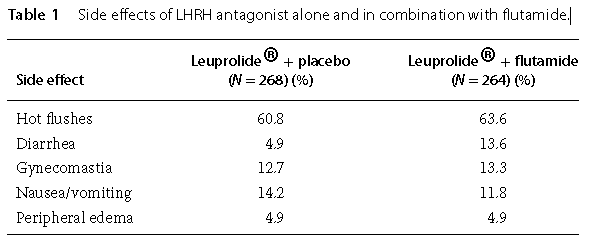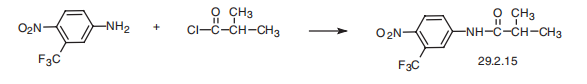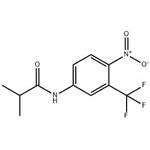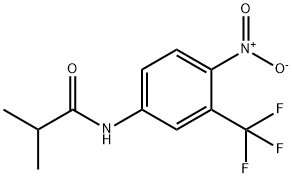
Flutamide
- Product NameFlutamide
- CAS13311-84-7
- CBNumberCB2468110
- MFC11H11F3N2O3
- MW276.21
- EINECS236-341-9
- MDL NumberMFCD00072009
- MOL File13311-84-7.mol
Chemical Properties
| Melting point | 112 °C |
| Boiling point | 400.3±45.0 °C(Predicted) |
| Density | 1.3649 (estimate) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, freely soluble in acetone and in ethanol (96 per cent). |
| pka | 13.12±0.70(Predicted) |
| color | Pale Yellow to Light Yellow |
| Merck | 14,4208 |
| InChI | InChI=1S/C11H11F3N2O3/c1-6(2)10(17)15-7-3-4-9(16(18)19)8(5-7)11(12,13)14/h3-6H,1-2H3,(H,15,17) |
| InChIKey | MKXKFYHWDHIYRV-UHFFFAOYSA-N |
| SMILES | C(NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1)(=O)C(C)C |
| CAS DataBase Reference | 13311-84-7(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | flutamide |
| FDA UNII | 76W6J0943E |
| NCI Drug Dictionary | flutamide |
| ATC code | L02BB01 |
| Proposition 65 List | Flutamide |
| EPA Substance Registry System | Flutamide (13311-84-7) |
Safety
| Symbol(GHS) |
  
|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H302-H351-H361-H373-H411 | |||||||||
| Precautionary statements | P202-P260-P264-P273-P301+P312-P308+P313 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 20/21/22-63-36/37/38 | |||||||||
| Safety Statements | 22-36-36/37/39-27-26 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | UG5700000 | |||||||||
| HS Code | 29242990 | |||||||||
| NFPA 704: |
|