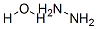Chemical Properties
colourless fuming liquid
Physical properties
Colorless fuming liquid; faint odor; refractive index 1.4284; density 1.032g/mL; boils at 119°C; solidifies at -51.7°C; miscible with water and alcohol;insoluble in chloroform, methylene chloride, and ether.
Uses
Hydrazine Hydrate used as a reactant in the cyclizations of pyridinones. It is also used in the study of nanocrystal semiconductors, participating in the functionalization and passivation of surface states. It is widely used as a reducing agent or an intermediate of synthesis in various industrial sectors like water treatment (effluents, industrial boilers), chemical treatment process (metals, mine extraction) or active ingredients synthesis (pharmaceuticals and agrochemicals).
Uses
Hydrazine hydrate is used as a reducing agent in synthetic and analytical reactions and as a solvent for many inorganic compounds. It also is used with methanol as a propellant for rocket engines. Another application is catalytic decomposition of hydrogen peroxide.
Preparation
Hydrazine hydrate is prepared by treating hydrazine sulfate, N2H4•H2SO4 with sodium hydroxide. The product is collected by distillation under nitrogen. It also is obtained as a by-product in the Bayer Ketazine process for producing hydrazine in which hydrazine solution is hydrolysed under pressure in a ketazine column.
Definition
ChEBI: Hydrazine hydrate is a hydrate. It contains a hydrazine.
General Description
A colorless fuming liquid with a faint ammonia-like odor. Corresponds to a 64% aqueous solution of hydrazine in water. Combustible but may require some effort to ignite. Contact with oxidizing materials may cause spontaneous ignition. Toxic by inhalation and by skin absorption. Corrosive to tissue. Produces toxics oxides of nitrogen during combustion.
Air & Water Reactions
Fumes in air. Water soluble.
Reactivity Profile
Hydrazine hydrate is a base and a very powerful reducing agent. Very corrosive. Violent reaction on contact with alkali metals (sodium, potassium), 2,4-dinitrochlorobenzene, tin dichloride, mercury oxide. Vigorous neutralization reaction with acids. Emits toxic fumes of nitrogen oxides when heated to decomposition [Lewis, 3rd ed., 1993, p. 680]. Reacts with tin(II) chloride to give tin(II) dihydrazine chloride, which decomposes explosively when heated [Mellor 7:430(1946-1947)]. Reacts exothermically and violently with 2,4-dinitrochlorobenzene [Wischmeyer (1967)].
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Purification Methods
It is best obtained by heating hydrazine sulphate (200g), NaOH (160g) and H2O (75mL, exothermic) in a copper flask under reflux for 1.5hours then distilled off (using a flame to remove all the hydrazine). The distillate (175mL) is a clear liquid which contains ~40-45% of N2H4. Note that hydrazine attacks glass, rubber and cork, and stainless steel equipment should be used. The percentage of hydrazine is determined by titration with standard acid (methyl orange indicator) or against standard iodine (starch indicator). Hydrazine monohydrate should contain 64% of N2H4. The ~40-45% solution may be concentrated by mixing it (144mL) with xylene (230mL) and distilling it through an efficient fractionating column (e.g. Hempel column, p 10). All the xylene passes over with about 85mL of H2O. On distilling the residue, hydrated hydrazine (50mL) is obtained containing 80-85% of N2H4. This can be diluted with conductivity H2O to 64% N2H4 to give the monohydrate. Hydrazine and its hydrates have VERY IRRITATING and TOXIC vapours and should be used in an efficient fume cupboard. [Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 469-472 1963.]