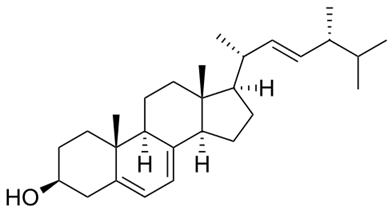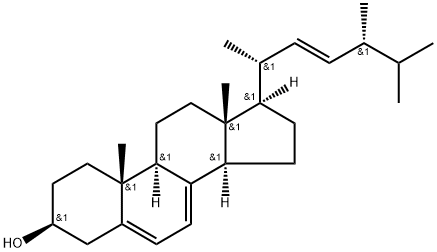
Ergosterol
- Product NameErgosterol
- CAS57-87-4
- CBNumberCB5763603
- MFC28H44O
- MW396.66
- EINECS200-352-7
- MDL NumberMFCD00003623
- MOL File57-87-4.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 156-158 °C(lit.) |
| alpha | -120 º (c=1, CHC13) |
| Boiling point | 250 °C (1.3 mmHg) |
| Density | 0.9784 (rough estimate) |
| refractive index | -112.5 ° (C=1, THF) |
| storage temp. | -20°C |
| solubility | Acetone (Slightly, Heated), Chloroform (Slightly), Ethyl Acetate (Slightly, Heated) |
| pka | 14.91±0.70(Predicted) |
| form | Crystalline Powder or Crystalline Needles |
| color | White to off-white |
| optical activity | [α]20/D 120±10°, c = 1% in chloroform |
| Water Solubility | PRACTICALLY INSOLUBLE |
| Sensitive | Light Sensitive |
| Merck | 14,3659 |
| BRN | 2338604 |
| Stability | Stable, but may be light or air sensitive. Incompatible with acids, strong oxidizing agents. |
| InChIKey | DNVPQKQSNYMLRS-APGDWVJJSA-N |
| LogP | 9.300 (est) |
Safety
| Symbol(GHS) |
 
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H413 | |||||||||
| Precautionary statements | P273 | |||||||||
| Hazard Codes | T+,T,Xn | |||||||||
| Risk Statements | 28-48/20/22-40-38-25-67-36/38-22-20-63 | |||||||||
| Safety Statements | 28-36/37-45-26 | |||||||||
| RIDADR | UN 2811 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 1-3-8-10 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29334900 | |||||||||
| Hazardous Substances Data | 57-87-4(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|