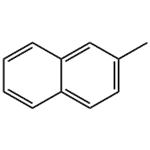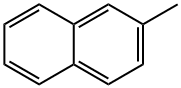
2-Methylnaphthalene
- Product Name2-Methylnaphthalene
- CAS91-57-6
- CBNumberCB5854626
- MFC11H10
- MW142.2
- EINECS202-078-3
- MDL NumberMFCD00004118
- MOL File91-57-6.mol
Chemical Properties
| Melting point | 34-36 °C(lit.) |
| Boiling point | 241-242 °C(lit.) |
| Density | 1.01 |
| vapor pressure | 5.4 (extrapolated, Mackay et al., 1982) |
| refractive index | 1.6019 |
| Flash point | 208 °F |
| storage temp. | 2-8°C |
| solubility | 0.025g/l |
| form | Crystalline Low Melting Solid |
| color | White |
| Odor | at 1.00 % in dipropylene glycol. sweet floral woody |
| Odor Type | floral |
| Water Solubility | 0.00246 g/100 mL |
| FreezingPoint | 34.5℃ |
| BRN | 906859 |
| Henry's Law Constant | 6.13 at 25 °C (thermodynamic method-GC/UV spectrophotometry, Altschuh et al., 1999) |
| Exposure limits | ACGIH: TWA 0.5 ppm (Skin) |
| Stability | Stable. Incompatible with strong oxidizing agents. |
Safety
| Symbol(GHS) |
 
|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335-H411 | |||||||||
| Precautionary statements | P261-P264-P273-P301+P312-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,N,T | |||||||||
| Risk Statements | 22-36/37/38-51/53-63-43-23/24/25-45-20/21/22-67-40 | |||||||||
| Safety Statements | 26-37/39-61-36/37-24/25-23-53-36-29 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | QJ9635000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29029080 | |||||||||
| Hazardous Substances Data | 91-57-6(Hazardous Substances Data) | |||||||||
| Toxicity | Acute oral LD50 for rats 1,630 mg/kg (quoted, RTECS, 1985). | |||||||||
| NFPA 704: |
|