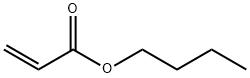
Butyl acrylate
- Product NameButyl acrylate
- CAS141-32-2
- CBNumberCB6100980
- MFC7H12O2
- MW128.17
- EINECS205-480-7
- MDL NumberMFCD00008809
- MOL File141-32-2.mol
- MSDS FileSDS
Chemical Properties
| Melting point | -69 °C |
| Boiling point | 61-63 °C60 mm Hg(lit.) |
| Density | 0.894 g/mL at 25 °C(lit.) |
| vapor density | >1 (vs air) |
| vapor pressure | 3.3 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point | 63 °F |
| storage temp. | Store below +30°C. |
| solubility | 1.7g/l |
| form | Liquid |
| color | Clear Colorless |
| Odor | Fruity |
| Odor Threshold | 0.00055ppm |
| explosive limit | 1.1-7.8%(V) |
| Relative density, gas (air=1) | 4.43 |
| Water Solubility | 1.4 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,1539 |
Safety
| Symbol(GHS) |
 
|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H226-H312+H332-H315-H317-H319-H335-H412 | |||||||||
| Precautionary statements | P210-P273-P280-P303+P361+P353-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | F,Xn,Xi | |||||||||
| Risk Statements | 11-20/21/22-37/38-43-52/53-36/37/38-10 | |||||||||
| Safety Statements | 16-25-37-61-9-36/37-26 | |||||||||
| RIDADR | UN 1993 3/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | UD3150000 | |||||||||
| F | 10 | |||||||||
| Autoignition Temperature | 559 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29161230 | |||||||||
| Hazardous Substances Data | 141-32-2(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in rats: 3.73 g/kg (Smyth) | |||||||||
| IDLA | 113 ppm | |||||||||
| NFPA 704: |
|


