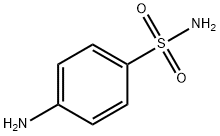
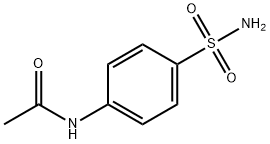
Product features
Sulfanilamide is an organic sulfur compound structurally similar to p-aminobenzoic acid (PABA) with antibacterial property. Sulfanilamide competes with PABA for the bacterial enzyme dihydropteroate synthase, thereby preventing the incorporation of PABA into dihydrofolic acid, the immediate precursor of folic acid. This leads to an inhibition of bacterial folic acid synthesis and de novo synthesis of purines and pyrimidines, ultimately resulting in cell growth arrest and cell death.Without it, bacteria cannot replicate.
- Mechanism of action: mechanism of action is to interfere with the synthesis of nucleic acids required for pathogenic microorganisms,making bacteria lack of nutrition and stop the growth, development and reproduction, having suppression killing effect on hemolytic streptococcus, staphylococcus and meningococcal.
- Pharmacodynamics: Oral easily absorbed from the gastrointestinal tract, widely distributed in the body, can penetrate the blood-brain barrier into the brain tissue, and can penetrate the placental barrier into the fetus. Rapid excretion, mainly excreted in metabolites from kidney.
- Clinical application: Mainly used for trauma infection caused by infection hemolytic streptococcus, staphylococcus, and local wound infections.
- Uses: Sulfanilamide is lower toxicity in sulfa drugs, can be applied for infants, pregnant women, pregnant women and during menstruation, but not in large doses. Having effects on hemolytic streptococcal infection (erysipelas, puerperal fever, tonsillitis), urinary tract infection (gonorrhea) and so; also the intermediate for synthesis of other sulfa drug (such as sulfa amidine, pyrimidine and sulfamethoxazole sulfa methoxy-triazine, etc. ).
Chemical properties
White granular or crystalline powder, odorless. Slightly bitter taste. Slightly soluble in water, ethanol, methanol, ether and acetone, soluble in boiling water, glycerol, hydrochloric acid, sodium hydroxide and potassium hydroxide solution, insoluble in chloroform, ether, benzene, petroleum ether.
Uses
- Sulfanilamide is the main raw material for the synthesis of sulfa drugs.
- Used as a reagent to determine nitrite, also used in the pharmaceutical industry.
- Used as intermediates for the synthesis of other sulfa drugs, even for wound disinfection.
- Amino benzene sulfonamide is intermediate of herbicide asulam, as well as intermediate of sulfa medicine.
- Veterinary medicine, topical anti-inflammatory drugs, for analysis and detection.
- Wide spectrum antibacterial, having antibacterial effects on hemolytic streptococcus, Neisseria meningitidis, Staphylococcus aureus and other Gram-positive and negative bacteria. This product is topical application, it can be partially absorbed from the wound. For trauma infections of hemolytic streptococcus and staphylococcus. It can also be used to quickly stop the bleeding wound.
Production methods
There are several methods for their preparation.
1. Acetyl aniline used as raw material
The acetanilide reacts with chlorosulfonic acid at 40~50 ℃, and then cooled slowly, added to water for acid decomposition, while precipitation, dried and filtered to give acetaminophen chloride and ammoniated, ammoniated temperature is controlled at 40~45 ℃, hydrolysis, acidification.
2. Method of mixed diphenyl urea
Condensation of aniline and urea is single-phenylurea and diphenyl urea (called mixed urea), and then obtained from chlorosulfonated, amination, hydrolysis, acid precipitation. The reaction procedure is as follows.
(1)Condensation condensation of aniline hydrochloride and urea, at a temperature of 101~110 ℃ reaction for 3~4 h, obtaining mixed diphenyl urea.
(2)Chlorosulfonated Chlorine acid is pressed into the sulfonated pot, stirring cooling, when the temperature drops below 10 ℃, uniformly added mixed phenyl urea under stirring, the reaction temperature is gradually increased, the addition is completed, at the 46~50 ℃ insulation and mixing for 2 h, cooled to below 10 ℃, added water for acid decomposition. Controlling that the decomposition temperature does not exceed 15 ℃, after the addition of water continued stirring for 20min, then by precipitation, washed with water, obtaining mixed phenylurea chloride.
(3)Ammoniated 2% aqueous ammonia is put into ammoniated pan, cooled to 25 ℃, stirring and added into a mixing phenyl urea chloride, control the temperature at 40 ℃, insulation and reaction for 3h, obtaining ammoniated liquid.
(4)Hydrolysis and neutralization The amide is heated up to 90 ℃, is added 3% lye, continue to heat 108~112 ℃, hydrolysis for 5 h, and moved in the crystallization pot, added hydrochloric acid to neutralize crystals and the crystals are cooled to 20 ℃, crystallization, filtration, washed with water, dry, products are obtained.
Description
Sulfanilamide is an organic sulfur compound structurally similar to p-aminobenzoic acid (PABA) with antibacterial property. Sulfanilamide competes with PABA for the bacterial enzyme dihydropteroate synthase, thereby preventing the incorporation of PABA into dihydrofolic acid, the immediate precursor of folic acid. This leads to an inhibition of bacterial folic acid synthesis and de novo synthesis of purines and pyrimidines, ultimately resulting in cell growth arrest and cell death.
Chemical Properties
White granules or powder crystals, odorless. Taste slightly bitter. Slightly soluble in cold water, ethanol, methanol, ether and acetone, easily soluble in boiling water, glycerin, hydrochloric acid, potassium hydroxide and sodium hydroxide solution, insoluble in chloroform, ether, benzene and petroleum ether.
Uses
Sulfanilamide is an antibacterial agent and antimicrobial agent of sulfonamide type (topical and vaginal).
Uses
Sulfonamide antibacterial Sulfanilamide is an antibacterial and used in the treatment of vaginal yeast infections. It is a competitive inhibitor of dihydropteroate synthestase to block the synthesis of folic acid. It serves as an intermediate for the preparation of 2,6-disubstituted anilines by electrophilic substitution followed by removal of the sulfonamide blocking group by desulfonation with sulfuric acid.
Preparation
Sulfonamide is synthesized from acetanilide by chlorosulfonation, amination, hydrolysis, and neutralization:
Acetanilide is reacted with chlorosulfonic acid at 40~50℃, then cooled, slowly added to water for acid decomposition, precipitated at the same time, dried and filtered to obtain p-acetamidobenzenesulfonyl chloride, and then subjected to ammoniation, and the amination temperature is controlled at 40~ 45 ℃, and then hydrolyzed, acidified to obtain sulfonamide.
Definition
ChEBI: Sulfanilamide is a sulfonamide in which the sulfamoyl functional group is attached to aniline at the 4-position. It has a role as an EC 4.2.1.1 (carbonic anhydrase) inhibitor, an antibacterial agent and a drug allergen. It is a substituted aniline, a sulfonamide antibiotic and a sulfonamide.
brand name
Acetonal vaginal;Amidrin;Avc cream suppositoty;Avc/dienestrol;Avril;Azol polvo;Azol pomada;Buco pental;Buco regis;Chemiovis;Daromid;Defonamid;Dorsec;Expseptoplix;Faderma;Fricton;Gagaril sulfamida;Gynaedron;Instilin;Jacosulfon;Medeyol;Mentol sedans sulfamidad;Nasopomada;Odamida;Oestro-gyneadron;Otocaina;Otonasal;Otorrilan;Ovuthricinol;Oxidermiol;Paraseptol;Pental forte;Pentalmicina;Polvo sulfamida leti;Polvo sulfamida orrvan;Polvos wilfe;Pomada heridas;Pomada wilfe;Prontablin;Pulvi bacteramide;Pyodental;Pyodron;Quimpeamida;Rhinamide;Rino glucol sulf;Sulfacromo;Sulfonamid spuman;Sulfonamide-spuman-style;Sulfonanilamid;Sulfosellan-salbe;Ung. vemleigh.
World Health Organization (WHO)
Sulfanilamide, a sulfonamide anti-infective agent, was introduced
in 1936 for the treatment of bacterial infections. The importance of sulfonamides
has subsequently decreased as a result of increasing resistance and their
replacement by antibiotics which are generally more active and less toxic. The
sulfonamides are known to cause serious adverse effects such as renal toxicity,
sometimes fatal exfoliative dermatitis and erythema multiforma and dangerous
adverse reactions affecting blood formation such as agranulocytosis and
haemolytic or aplastic anaemia. Sulfanilamide is still used in some countries as a
pessaries or as vaginal cream.
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 9, p. 71, 1961
DOI: 10.1248/cpb.9.71
Antimicrobial activity
Sulfanilamide is a sulfonamide antibiotic. It is bacteriostatic against Streptococci in vitro at a concentration of 20 μg/ml and inhibits the growth of 106 clinically isolated strains of Gonococcus. Sulfanilamide reduces the concentration of Streptococcus in rabbit plasma ex vivo following four doses of 20 ml of a 2% sulfanilamide solution. Sulfonamide class antibiotics, of which sulfanilamide is a member, are bacteriostatic and inhibit bacterial synthesis of dihydrofolic acid by competing with 4-aminobenzoic acid for binding to dihydropteroate synthase. Formulations containing sulfanilamide have been used to treat T. vaginalis infections.
General Description
White powder. pH of 0.5% aqueous solution: 5.8-6.1.
Air & Water Reactions
May be unstable if exposed for long periods air and light . Slightly water soluble.
Reactivity Profile
Sulfanilamide is an amino acid. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. May react with azo and diazo compounds to generate toxic gases.
Fire Hazard
Flash point data for Sulfanilamide are not available but Sulfanilamide is probably combustible.
Biochem/physiol Actions
Sulfonamide antibiotic that blocks the synthesis of dihydrofolic acid by inhibiting the enzyme dihydropteroate synthase.Mode of Action: A competitive inhibitor of dihydropteroate synthestase to block the synthesis of folic acid.Anti-microbial Spectrum: Gram positive, Gram negative, Chlamydia Mode of Resistance: Alteration of dihydropteroate synthase or alternative pathway for folic acid synthesis.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion, subcutaneous, and intravenous routes. Human teratogenic effects by unspecified route: developmental abnormalities of the blood and lymphatic systems (including the spleen and bone marrow). Experimental reproductive effects. Questionable carcinogen with experimental carcinogenic data. Mutation data reported. Implicated in aplastic anemia. When heated to decomposition it emits very toxic fumes of NOx and SOx.