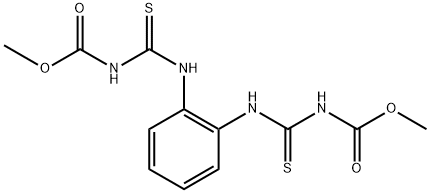
Description
Thiophanate methyl is a member of benzimidazole and a systemic fungicide with protective and curative activity against a broad spectrum of fungal diseases including leaf spots, blotches, blights, fruit spots, rots, sooty mould, scabs, bulb/corn/tuber decays, blossom blights, powdery mildews, certain rusts, and common soil borne crown and root rots. It is used on a variety of tree, vine, root crops, canola, and wheat, as well as on lawns and ornamentals. Thiophanate methyl is absorbed by the roots and leaves. Thiophanate-methyl has a low mammalian toxicity. But, it is an irritant and a skin sensitizer. It may also be mutagen. Moderate toxicity of thiophanate methyl is observed for aquatic organisms and earthworms. In EU, thiophanate methyl is approved to control fungal diseases on crops, while in Australia it is not registered for use on food-producing species, while it is only for the control of soil-borne diseases of ornamentals plants. For application, Thiophanate-methyl is formulated into wettable powders containing 50 and 70% active ingredient.
References
- https://apvma.gov.au
- https://www.epa.gov
- https://sitem.herts.ac.uk
- http://www.inchem.org
- Terry R Roberts, David H Hutson, Philip W Lee, Peter H Nicholls, and Jack R Plimmer, Metabolic Pathways of Agrochemicals: Part 2: Insecticides and Fungicides, 1999, ISBN 978-0-85404-499-3
Chemical Properties
colourless crystals, or white or light brown powder
Chemical Properties
Methyl thiophanate is a colorless crystals, prisms or light brown powder.
Uses
Thiophanate methyl is a fungicide/wound protectant used to control
plant diseases in stone fruit, pome fruit, tropical and subtropical fruit
crops, grapes and fruiting vegetables. Thiophanate methyl is effective
against a wide variety of fungal diseases such as leaf spots, blotches and
blights; fruit spots and rots; sooty mould; scabs; bulb, corn and tuber
decays; blossom blights; powdery mildews; certain rusts; and common
soil borne crown and root rots.
Uses
Thiophanate-Methyl is a systematic fungicide of the thiophanate class. Thiophanate-Methyl is commonly used in agriculture to protect against powdery mildew, rot and other fungal diseases in fruits, ve
getables and other crops.
Definition
ChEBI: A member of the class of thioureas that is the dimethyl ester of (1,2-phenylenedicarbamothioyl)biscarbamic acid. A fungicide effective against a broad spectrum of diseases in fruit, vegetables, turf and other crops including eyespot, scab, powdery mildew a
d grey mould.
General Description
Colorless crystals or light brown powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Thiophanate-methyl is incompatible with strong acids and bases, and with strong reducing agents such as hydrides. Produces flammable gaseous hydrogen with active metals or nitrides. Incompatible with strong oxidizing acids, peroxides. Incompatible with alkaline material. Forms complexes with copper salts .
Fire Hazard
Flash point data for Thiophanate-methyl are not available. Thiophanate-methyl is probably combustible.
Flammability and Explosibility
Not classified
Agricultural Uses
Fungicide: Thiophanate-methyl is a systemic fungicide used to
control a broad spectrum of fungal diseases on fruits, vegetables,
turf and ornamentals, including shade trees, and diseases
in the field, nurseries, and in greenhouses. Note: Do not
confuse with thiophanate-ethyl (CAS: 23564-06-9), which is
not currently registered for use in the U.S. Registered for use
in EU countries. Registered for use in the U.S.
Trade name
BASF® 32500 F; BASF® 32500 Fungicide;
CERCOBIN®; CLEARY® 3336; CONSYST®;
DITEK®[C]; DOMAIN®; DOUSAN®[C]; ENOVIT®;
EVOLVE®; FANATE®; FUNGITOX®; FUNGO®;
MILDOTHANE® Turf Liquid; NEOTOPSIN®; NF-
35®; NF-44®; NSC 170810®; PELT®; PRO-TURF®;
SIPCAVIT®; SNARE®; SPECTRO®; SYSTEC®;
SYSTEMIC® FUNGICIDE; TD 1771®; TOPSIN®;
TOPSIN-WP METHYL®; 3336 TURF FUNGICIDE®;
ZYBAN®
Potential Exposure
Methyl thiophanate is thiocarbamate ester, used in the synthesis of polymers and in agriculture as pesticides, soil fumigants, and seed disinfectants.
Carcinogenicity
Those long-term studies evaluated a
variety of toxicity end points including carcinogenicity.
Both benomyl and carbendazim were weak carcinogens in
some in vivo studies. Rats of the SpDxAug strain were fed 80,
400, and 2000 ppm for 2 years and no evidence for an
increase in tumors was seen. In carcinogenicity
studies in mice with carbendazim, similar liver tumor finding
were found in CD-1 or Swiss strains. On the other
hand, no evidence of tumors was seen in a similar study
conducted using the NMRKf mouse strain, a strain that has a
low incidence of spontaneous tumors.
Metabolic pathway
Thiophanate methyl degradation in water (hydrolytic/ photolysis), soils,
plants and animals follows a common pathway that involves the initial
conversion into methyl benzimidazol-2-ylcarbamate (carbendazim).
Prior to benzimidazole ring formation, at least one side chain undergoes
an oxidative desulfuration reaction and partial hydrolysis (even to
the substituted aniline). Once formed, carbendazim is degraded primarily
via an oxidative mechanism (in animals) or through hydrolysis
to 2-aminobenzimidazole (in soils). Possible metabolism pathways of
thiophanate methyl are depicted in Scheme 1.
Shipping
UN2771 Thiocarbamate pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Degradation
Thiophanate methyl (1) is susceptible to alkaline hydrolysis. The principal
degradation product is carbendazim (2). Thiophanate methyl degraded
slowly in water (pH 7-7.4, Noguchi et al., 1971), and in fruit juice (grape
homogenate, Shiga et al., 1985) to primarily carbendazim, but also to 1-(3-
methoxycarbony1thioureido)-2-(3-methoxycarbony1ureido)benzene (3)
and dimethyl 4,4'-(o-phenylene)bis(allophanate) (4).
Thiophanate methyl underwent photolysis in aqueous solution to
carbendazim (DT
50 <1 day) when illuminated with UV light (254 nm)
or natural sunlight. A similar photolytic transformation occurred on
moist cotton foliage, although no photolysis occurred when thiophanate
methyl was exposed as a dry film to light (Buchenauer et al., 1973).
Thiophanate methyl on wet filter paper decomposed in sunlight to yield
carbendazim and small amounts of compounds 3 and 4 (Ono and
Tohyama, 1982).
Incompatibilities
Thiocarbamate esters are combustible; dust may form explosive mixture with air. Decomposes163℃Thiocarbamate esters are combustible. They react violently with powerful oxidizers such as calcium hypochlorite. Poisonous gases are generated by the thermal decomposition of thiocarbamate compounds, including carbon disulfide, oxides of sulfur, oxides of nitrogen, hydrogen sulfide, ammonia, and methylamine. Thio and dithio carbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids. Flammable gases are generated by the combination of thiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates are incompatible with acids, peroxides, and acid halides.
Waste Disposal
In accordance with 40CFR 165 recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.