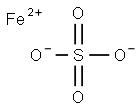
FERROUS SULFATE
- Product NameFERROUS SULFATE
- CAS7720-78-7
- CBNumberCB7450570
- MFFeO4S
- MW151.91
- EINECS231-753-5
- MDL NumberMFCD00011014
- MOL File7720-78-7.mol
Chemical Properties
| Melting point | decomposes at 671℃ [JAN85] |
| Density | 3.650 |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | Store at +15°C to +25°C. |
| solubility | Water (Slightly) |
| form | white orthorhombic crystals |
| color | white orthorhombic crystals, crystalline; hygroscopic |
| Odor | at 100.00?%. odorless |
| Water Solubility | g/100g solution H2O: 13.6 (0°C), 22.8 (25°C), 24.0 (100°C); solid phase, FeSO4 · 7H2O (0°C, 25°C), FeSO4 ·H2O (100°C) [KRU93] |
| Dielectric constant | 14.2(14℃) |
| FDA 21 CFR | 184.1315; 582.5315; 310.545; 582.80 |
| SCOGS (Select Committee on GRAS Substances) | Ferrous sulfate Ferrous sulfate (packaging) |
| CAS DataBase Reference | 7720-78-7(CAS DataBase Reference) |
| EWG's Food Scores | 1-5 |
| NCI Dictionary of Cancer Terms | ferrous sulfate |
| FDA UNII | 2IDP3X9OUD |
| NCI Drug Dictionary | ferrous sulfate |
| ATC code | B03AA07,B03AD03 |
Safety
| Symbol(GHS) |

|
| Signal word | Warning |
| Hazard statements | H302-H315-H319 |
| Precautionary statements | P264-P270-P301+P312-P330-P501-P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P |
| Risk Statements | 25 |
| Safety Statements | 45 |
| HS Code | 28332910 |
| Hazardous Substances Data | 7720-78-7(Hazardous Substances Data) |
| Toxicity | LD50 oral in rat: 319mg/kg |


