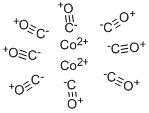
Description
Cobalt carbonyl (known as dicobalt octocarbonyl) is a versatile reagent and catalyst in organometallic chemistry and organic synthesis. It has catalytic applications in various chemical reactions such as hydroformylation of unsaturated compound, homogeneous hydrogenation of aromatic hydrocarbons, hydrosilation of alkenes, reactions of disulfides such as carbonylation to thio-esters and desulfurization to sulfides, hydroformylation as well as the conversion of alkenes into aldehydes. It can also promote both the Pauson-Khand reactions and Nicholas reaction.

Reaction
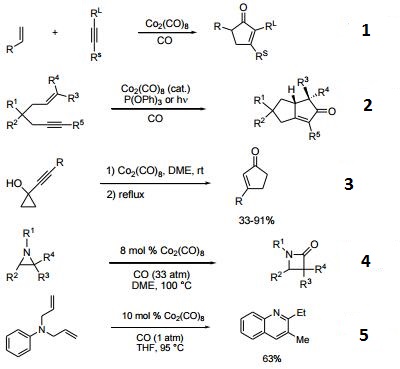
- Reagent for the Pauson-Khand conversion of an olefin, an alkyne and carbon monoxide into a cyclopentenone.
- Precatalyst in combination with triphenylphosphite for the cataytic Pauson-Khand reaction.
- Catalyzes the rearrangement of 1-alkynylcyclopropanols to cyclopentenones.
- Catalyzes the conversion of aziridines to β-lactams.
- Catalyzes the conversion of diallylanilines and aryliminies to quinolines.
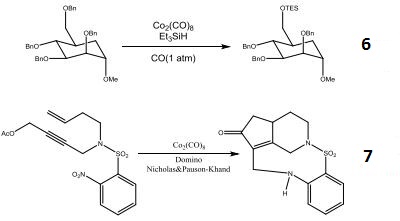
- Reagent for the selective cleavage of benzyl ethers.
- Domino Nicholas and Pauson-Khand process induced by nitroarene reduction.
Sources
Adkins, Homer., and G. Krsek. JACS 71.9(1949):3051-3055.
Feder, Harold M., and J. Halpern. Cheminform 7.7(1976):no-no.
Seitz, Friedrich, and M. S. Wrighton. Angewandte Chemie International Edition 27.2(1988):289-291.
Antebi, Shlomo, and H. Alper. Tetrahedron Letters 16.38(1985):no-no.
Krafft, Marie E., L. V. R. Boñaga, and C. Hirosawa. Cheminform 32.37(2001):no-no.
https://en.wikipedia.org/wiki/Dicobalt_octacarbonyl
Description
Cobalt carbonyl is a pyrophoric (spontaneously flammable in air), red-orange (when pure) to darkbrown crystalline solid. Molecular weight =341.94; Boilingpoint =(decomposes) 52℃; Freezing/Melting point =(decomposes) 50.5℃; Vapor pressure = 0.7 mmHg at 25℃.Hazard Identification (based on NFPA-704 M RatingSystem): Health 3, Flammability 3, Reactivity 2 .Insoluble in water.
Chemical Properties
Cobalt carbonyl is a pyrophoric (spontaneously flammable in air), red-orange (when pure) to dark-brown crystalline solid.
Chemical Properties
red-orange to dark red crystals or flakes
Physical properties
Orange crystals; density 1.78 g/cm
3; melts at 5l°C; decomposes above this temperature; insoluble in water; soluble in most organic solvents including alcohol, ether, carbon disulfide.
Uses
The use of dicobalt octacarbonyl as a catalyst in a variety of organic syntheses has led to the study of an extensive and important organometallic chemistry of cobalt.
Uses
It is used as a catalyst in many organicconversion reactions, which include hydrogenation,isomerization, hydroformylation,polymerization, and carbonylation.
Uses
- Cobalt carbonyl [Co2(CO)8] is commonly used as a catalyst in the hydroformylation (oxo reaction) of alkenes.
- Along with pyridine, it can be used as a catalyst in the carboxylation of alkenes into corresponding acids and esters.
- It is employed as a key precursor in the preparation of cobalt platinum (CoPt3), cobalt sulfide (Co3S4) and cobalt selenide (CoSe2) nanocrystals.
- It is also used as a reagent in Pauson-Khand cyclizations and Nicholas reaction.
Uses
Cobalt octacarbonyl is used as a catalyst in the Oxo process. It also is used as a catalyst for hydrogenation, isomerization, hydrosilation and polymerization reactions. The compound is also a source of producing pure cobalt metal and its purified salts.
Preparation
Cobalt octacarbonyl is prepared by the reaction of finely divided cobalt with carbon monoxide under pressure:
2Co + 8CO → Co
2(CO)
8
The compound may be prepared in a similar way from cobalt(II) iodide. Also, it may be prepared by thermal decomposition of cobalt carbonyl hydride:
2HCo(CO)
4 → Co
2(CO)
8 + H
2
Hazard
Toxic by ingestion and inhalation.
Health Hazard
Dicobalt octacarbonyl exhibits moderate toxicityby inhalation route and somewhatlower toxicity by intraperitoneal and oralroutes. However, it is much less toxicthan nickel tetracarbonyl or iron pentacarbonyl.A 2-hour LC50 value in mice isreported as 27 mg/m
3 (Lewis 1996). Anoral LD50 value in rats is within the rangeof 750–800 mg/kg. It decomposes, evolvingtoxic carbon monoxide.
Safety Profile
Poison by inhalation
and intraperitoneal routes. Questionable
carcinogen. Decomposes in air to form a
product that ignites spontaneously in air.
"hen heated to decomposition it emits
acrid smoke and fumes. See also
CARBONYLS and COBALT
COMPOUNDS.
Potential Exposure
This material is used as a catalyst for a number of reactions. It is also used in antiknock gasoline and for high-purity cobalt salts.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
storage
Color Code—Red Stripe: Flammability Hazard:Store separately from all other flammable materials. Priorto working with this chemical you should be trained on itsproper handling and storage. Decomposes on exposure toair or heat; stable in atmosphere of hydrogen and carbonmonoxide. Store in airtight, unbreakable containers in acool, well-ventilated area away from strong oxidizers andacids.
Shipping
UN3124 Toxic solids, self-heating, n.o.s., Hazard Class: 6.1; 6.1-Poisonous materials, 4.2-Spontaneously combustible material. Technical Name Required. UN3190 Self-heating solid, inorganic, Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible material, Technical Name Required. UN1325 Flammable solid, organic, n.o.s. Hazard Class: 4.1; Labels: 4.1-Flammable solid
Purification Methods
It forms orange-brown crystals on recrystallisation from n-hexane under a carbon monoxide atmosphere [Ojima et al. J Am Chem Soc 109 7714 1987; see also Hileman in Preparative Inorganic Reactions, Ed. Jolly, Vol 1 p 101 1987].
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Decomposes on exposure to air or heat (@ ~52°C) producing toxic fumes of cobalt and oxides of carbon