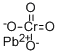Yellow monoclinic crystals; refractive index 2.31; density 6.12 g/cm3; melts at 844°C; decomposes on further heating; insoluble in water; also insoluble in ammonia and acetic acid; soluble in acids and alkalies.

Lead chromate is used in the production of commercial lead chromate pigments, such as Primrose Chrome Yellow, Light Chrome Yellow and Medium Chrome Yellow, containing 65-89% lead chromate (IARC 1990).
Lead chromate(VI) is also used in the preparation of green pigments such as chrome green or green cinnabar, in which it is mixed with Prussian blue (qq.v.). It is also listed by the Colour Index as a component of Pigment Green 15.
Lead chromate occurs in nature as the minerals, crocoite and phoenicochroite. It is an important pigment of lead used in oil paints and water colors. It is used for printing textiles and coloring porcelain.
Lead chromate(VI), PbCrO4, also known as plumbous chromate, was first synthesised around 1800, with its potential use as a pigment first reported by Berthollet and Vauquelin in 1804. According to Vauquelin (1809), it was prepared by adding lead acetate or lead nitrate to potassium chromate, and the shade of the precipitate could be varied (from yellow to orange or red) by adjusting the acidity/alkalinity of the products. Lead chromate( VI), which occurs naturally as the rare mineral crocoite (Rutley, 1988), is known as the pigment chrome yellow (qq.v.). It is listed by the Colour Index (1971) as CI Pigment Yellow 34 (CI 77600). The commercial manufacture of the pigment was largely influenced by the availability of suitable raw materials (usually in the form of the abundant mineral chromite, FeCr2O4). The earliest record of the use of chrome yellow is in a painting by Sir Thomas Lawrence dated prior to 1810 (Kühn, 1969). Sources of the pigment are reported by Harley (1982, citing Field, 1809) to be available by 1814-15, with Bollman (1769-1821) cited as the first commercial manufacturer in England, with production beginning around 1814-16.
Lead chromate is found naturally in minerals crocoite and phoenicochroite. It also is readily prepared by adding a soluble chromate such as sodium or potassium chromate to a solution of lead nitrate, lead acetate or other soluble lead(II) salt in neutral or slightly acidic solution:
Pb2+ + CrO42¯ → PbCrO4
The yellow precipitate is filtered, washed and dried.
Moderately toxic by intraperitoneal route. The effects, however, are mild from oral intake. Occupational exposure may cause cancer. There is sufficient evidence of carcinogenicity in animals and humans.
Lead chromate is an orange or orange-yellowcrystalline solid or powder. Molecular weight = 323.19;546.38 (lead chromate oxide); Boiling point = (decom-poses);Freezing/Melting point = 844℃. HazardIdentification (based on NFPA-704 M Rating System):Health 3, Flammability 0, Reactivity 0. Insoluble in water.
Lead chromate is an orange or orange-yellow
crystalline solid or powder.
It is used in chemical analysis of organic substances. pigment for paints and inks; in oil and water colors; printing fabrics, decorating china and porcelain; in chemical analysis of organic substances; in traffic paints.
Lead chromate (PbCrO4) is found in nature as yellow crystals in the mineral crocoite. It
can be produced by reacting lead chloride and sodium dichromate. It is a popular and safe
yellow pigment.
Lead chromate, “chrome yellow” PbCrO4, yellow precipitate, by reaction of soluble lead salt solution and sodium dichromate or chromate solution.Used as a pigment; basic lead chromate, red solid, insoluble, formed by heating lead chromate and sodium hydroxide solution.
ChEBI: A chromium coordination entity comprising chromate and lead(2+) ions in a 1:1 ratio
Lead chromate is a suspected
human lung carcinogen and can cause chronic
lead poisoning.
Lead chromate is used to make paint
pigments for wood and metal
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immedi-ately with soap and water. Seek medical attention immedi-ately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precau-tions, including resuscitationmask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quanti-ties of water and induce vomiting. Do not make an uncon-scious person vomit.Antidotes and special procedures for lead: Persons with sig-nificant lead poisoning aresometimestreated with CaEDTA while hospitalized. This“chelating” drug causes arush of lead from the body organs into the blood and kid-neys, and thus has its own hazards, and must be adminis-tered only by highly experienced medical personnel undercontrolled conditions and careful observation. Ca EDTA orsimilar drugs should never be used to prevent poisoningwhile exposure continues or without strict exposure control,as severe kidney damage can result.Note to physician: For severe poisoning BAL [BritishAnti-Lewisite, dimercaprol, dithiopropanol (C3HgOS2)] hasbeen used to treat toxic symptoms of certain heavy metalspoisoning. In the case of lead poisoning it may haveSOME value. Although BAL is reported to have a largemargin of safety, caution must be exercised, because toxiceffects may be caused by excessive dosage. Most can beprevented by premedication with 1-ephedrine sulfate(CAS: 134-72-5).
Color Code- Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. I ead chromate must be stored to avoid contactwith oxidizers (such as perchlorates, peroxides, permanga-nates,chlorates, and nitrates) andchemically active metals(such as potassium, sodium, magnesium, zinc and ferricferrocyanide), since violent reactions occur. A regulated,marked area should be established where this chemical is handled,used, or stored in compliance with OSHAStandard 1910. 1045.
UN3085 Oxidizing solid, corrosive, n.o.s,
Hazard Class: 5.1; Labels: 5.1-Oxidizer, 8-Corrosive material, Technical Name Required.
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides, and active metals; hydrazine, sodium, and potassium; organics at elevated temperature. Reacts with aluminum dinitronaphthalene, iron(III)
hexacyanoferrate (IV)