L-Malic acid is nearly odorless (sometimes a faint, acrid odor) with
a tart, acidic taste. It is nonpungent. May be prepared by hydration
of maleic acid; by fermentation from sugars.
L-Malic acid is nearly odorless (sometimes a faint, acrid odor). This compound has a tart, acidic, nonpungent taste.
clear colourless solution
Occurs in maple sap, apple, melon, papaya, beer, grape wine, cocoa, sake, kiwifruit and chicory root.
The naturally occuring isomer is the L-form which has been found in apples and many other fruits and plants. Selective α-amino protecting reagent for amino acid derivatives. Versatile synthon for the preparation of chiral compounds including κ-opioid rece
L-Malic acid is used as a food additive, Selective α-amino protecting reagent for amino acid derivatives. Versatile synthon for the preparation of chiral compounds including κ-opioid receptor agonists, 1α,25-dihydroxyvitamin D3 analogue, and phoslactomycin B.
Intermediate in chemical synthesis. Chelating and buffering agent. Flavoring agent, flavor enhancer and acidulant in foods.
L-Malic acid can be prepared by hydration of maleic acid; by fermentation from sugar.
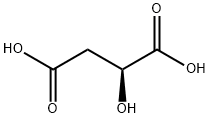
ChEBI: An optically active form of malic acid having (S)-configuration.
L-Malic acid is an organic acid that is commonly found in wine. It plays an important role in wine microbiological stability.
L-Malic acid is a part of cellular metabolism. Its application is recognized in pharmaceutics. It is useful in the treatment of hepatic malfunctioning, effective against hyper-ammonemia. It is used as a part of amino acid infusion. L-Malic acid also serves as a nanomedicine in the treatment of brain neurological disorders. A TCA (Krebs cycle) intermediate and partner in the malic acid aspartate shuttle.
Crystallise S-malic acid (charcoal) from ethyl acetate/pet ether (b 55-56o), keeping the temperature below 65o. Or dissolve it by refluxing in fifteen parts of anhydrous diethyl ether, decant, concentrate to one-third volume and crystallise it at 0o, repeatedly to constant melting point. [Beilstein 3 IV 1123.]