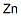Chemical Properties
| Melting point | 420 °C(lit.) |
| Boiling point | 907 °C(lit.) |
| Density | 7.14 g/mL at 25 °C |
| vapor pressure | 1 mm Hg ( 487 °C) |
| Flash point | 1 °F |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | wire |
| Specific Gravity | 7.14 |
| color | Silvery-gray |
| Odor | at 100.00?%. odorless |
| Resistivity | 5.8 μΩ-cm, 20°C |
| Water Solubility | Soluble in water. |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,10132 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Stability | Stable. Incompatible with amines, cadmium, sulfur, chlorinated solvents, strong acids, strong bases. Air and moisture sensitive. Zinc powder is very flammable. |
| InChIKey | HCHKCACWOHOZIP-UHFFFAOYSA-N |
| CAS DataBase Reference | 7440-66-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Zinc(7440-66-6) |
| EPA Substance Registry System | Zinc (7440-66-6) |
Safety Information
| Hazard Codes | N,F,Xi,Xn |
| Risk Statements | 52/53-50/53-17-15-36/37/38-51/53-36/37-22-19-40-11 |
| Safety Statements | 26-61-60-46-43-36-36/37-16 |
| RIDADR | UN 3264 8/PG 3 |
| WGK Germany | 3 |
| RTECS | ZH1400000 |
| F | 3 |
| Autoignition Temperature | 460 °C |
| TSCA | Yes |
| HS Code | 7904 00 00 |
| HazardClass | 8 |
| PackingGroup | III |
| Hazardous Substances Data | 7440-66-6(Hazardous Substances Data) |
| Toxicity | Zinc is an essential nutrient and is not regarded as toxic. However, the metal fumes, its oxide fumes, and chloride fumes can produce adverse inhalation effects. (See Zinc Oxide and Zinc Chloride, Toxicity) Ingestion of soluble salts can cause nausea. |
MSDS
| Provider | Language |
|---|---|
| ACROS | English |
| SigmaAldrich | English |
| ALFA | English |
Usage And Synthesis
Zinc (Zn) is a metallic element discovered by a German
chemist, Andreas Marggraf, in 1746. It is environmentally
ubiquitous and essential for life. It exhibits a strong tendency to
react with both organic and inorganic compounds. Zinc is the
24th most abundant element, constitutes 0.027% bw of the
Earth’s crust, and has five stable isotopes. The largest mineable
amounts are found in Australia, Asia, and the United States. A
recent estimate suggests that approximately 20% of the world’s
population is at risk of Zn deficiency. However, free access to
uncontrolled amounts of Zn in nutritional supplements is the
most common cause of Zn excess. Both Zn deficiency and Zn
excess contribute to human Zn toxicity.
Zinc is a soft silvery colored metal; the dust is odorless and gray. It is one of the most common elements in the earth's crust. Metal zinc was first produced in India and China during the middle ages. Industrially important compounds of zinc are zinc chloride (ZnCl2), zinc oxide (ZnO), zinc stearate (Zn(C16H35O2)2), and zinc sulfide (Sphalerite, ZnS) found in hazardous waste sites. It is found in air, soil, and water, and is present in all foods.

Pure zinc is a bluish-white shiny metal. Zinc has many commercial uses as coatings to prevent rust, in dry-cell batteries, and mixed with other metals to make alloys like brass and bronze. Zinc combines with other elements to form zinc compounds. Zinc compounds are widely used in industry to make paint, rubber, dye, wood preservatives, and ointments.

Pure zinc is a bluish-white shiny metal. Zinc has many commercial uses as coatings to prevent rust, in dry-cell batteries, and mixed with other metals to make alloys like brass and bronze. Zinc combines with other elements to form zinc compounds. Zinc compounds are widely used in industry to make paint, rubber, dye, wood preservatives, and ointments.
Bluish-white lustrous metal; brittle at room temperature; malleable between 100 to 150°C; hexagonal close-packed structure; density 7.14 g/cm3; melts at 419.6°C; vaporizes at 907°C; vapor pressure 1 torr at 487°C, 5 torr at 558°C and 60 torr at 700°C; good conductor of electricity, electrical resistivity 5.46 microhm-cm at 0°C and 6.01 microhm-cm at 25°C; surface tension 768 dynes/cm at 600°C; viscosity 3.17 and 2.24 centipoise at 450 and 600°C, respectively; diamagnetic; magnetic susceptibility 0.139x10–6 cgs units in polycrystalline form; thermal neutron absorption cross-section 1.1 barns.
Zinc is a whitish metal with a bluish hue. As an electropositive metal, it readily gives up itstwo outer electrons located in the N shell as it combines with nonmetal elements. Zinc foilwill ignite in moist air, and zinc shavings and powder react violently with acids. Zinc’s meltingpoint is 419.58°C, its boiling point is 907°C, and its density is 7.14 g/cm3.Note: Zinc is not always included as one of the metals in the first series of the transitionelements, but it is the first element in group 12 (IIB).
There are 38 isotopes of zinc, ranging in atomic weights from Zn-54 to Zn-83.Just four of these are stable, and those four, plus one naturally radioactive isotope (Zn-70) that has a very long half-life (5×10+14 years), make up the element’s existence onEarth. Their proportional contributions to the natural existence of zinc on Earth are assuch: Zn-64 = 48.63%, Zn-66 = 27.90%, Zn-67 = 4.10%, Zn- 68 = 18.75%, and Zn-70 = 0.62%. All the other isotopes are radioactive and artificially produced.
Although ancients used zinc compounds, the name “zinc” is assumed
to be derived from the German word zinn, which was related to tin.
Zinc is the 24th most abundant on Earth, which means it makes up only about 0.007%of the Earth’s crust. Even so, humans have found many uses for it over the past thousands ofyears.It is not found in its pure metallic form in nature but is refined from the mineral (compound) zinc sulfide (ZnSO4) known as the ores sphalerite and zincblende. It is also recoveredfrom minerals and ores known as willemite, hydrozincite, smithsonite, wurtzite, zincite, andFranklinite. Zinc ores are found in Canada, Mexico, Australia, and Belgium, as well as in theUnited States. Valuable grades of zinc ores are mined in Colorado and New Jersey.
Zinc is malleable and can be machined, rolled, die-cast, molded into various forms similarto plastic molding, and formed into rods, tubing, wires, and sheets. It is not magnetic, butit does resist corrosion by forming a hard oxide coating that prevents it from reacting anyfurther with air. When used to coat iron, it protects iron by a process called “galvanic protection,” also known as “sacrificial protection.” This protective characteristic occurs because theair will react with the zinc metal coating, which is a more electropositive (reactive) metal thanis the coated iron or steel, which is less electropositive than zinc. In other words, the zinc isoxidized instead of the underlying metal. (See the section under “Common Uses of Zinc” formore on galvanization.
Centuries before zinc was recognized as a distinct element,
zinc ores were used for making brass. Tubal-Cain, seven generations
from Adam, is mentioned as being an “instructor in
every artificer in brass and iron.” An alloy containing 87%
zinc has been found in prehistoric ruins in Transylvania.
Metallic zinc was produced in the 13th century A.D. in India
by reducing calamine with organic substances such as wool.
The metal was rediscovered in Europe by Marggraf in 1746,
who showed that it could be obtained by reducing calamine
with charcoal. The principal ores of zinc are sphalerite or
blende (sulfide), smithsonite (carbonate), calamine (silicate),
and franklinite (zinc, manganese, iron oxide). Canada, Japan,
Belgium, Germany, and the Netherlands are suppliers of zinc
ores. Zinc is also mined in Alaska, Tennessee, Missouri, and
elsewhere in the U.S. Zinc can be obtained by roasting its
ores to form the oxide and by reduction of the oxide with coal
or carbon, with subsequent distillation of the metal. Other
methods of extraction are possible. Naturally occurring zinc
contains five stable isotopes. Twenty-five other unstable
isotopes and isomers are recognized. Zinc is a bluish-white,
lustrous metal. It is brittle at ordinary temperatures but malleable
at 100 to 150°C. It is a fair conductor of electricity, and
burns in air at high red heat with evolution of white clouds
of the oxide. The metal is employed to form numerous alloys
with other metals. Brass, nickel silver, typewriter metal,
commercial bronze, spring brass, German silver, soft solder,
and aluminum solder are some of the more important alloys.
Large quantities of zinc are used to produce die castings,
used extensively by the automotive, electrical, and hardware
industries. An alloy called Prestal?, consisting of 78% zinc
and 22% aluminum, is reported to be almost as strong as
steel but as easy to mold as plastic. It is said to be so plastic
that it can be molded into form by relatively inexpensive die
casts made of ceramics and cement. It exhibits superplasticity.
Zinc is also extensively used to galvanize other metals
such as iron to prevent corrosion. Neither zinc nor zirconium
is ferromagnetic; but ZrZn2 exhibits ferromagnetism
at temperatures below 35 K. Zinc oxide is a unique and very
useful material to modern civilization. It is widely used in the
manufacture of paints, rubber products, cosmetics, pharmaceuticals,
floor coverings, plastics, printing inks, soap,
storage batteries, textiles, electrical equipment, and other
products. It has unusual electrical, thermal, optical, and solid-
state properties that have not yet been fully investigated.
Lithopone, a mixture of zinc sulfide and barium sulfate, is
an important pigment. Zinc sulfide is used in making luminous
dials, X-ray and TV screens, and fluorescent lights. The
chloride and chromate are also important compounds. Zinc
is an essential element in the growth of human beings and
animals. Tests show that zinc-deficient animals require 50%
more food to gain the same weight as an animal supplied
with sufficient zinc. Zinc is not considered to be toxic, but
when freshly formed ZnO is inhaled a disorder known as the
oxide shakes or zinc chills sometimes occurs. It is recommended
that where zinc oxide is encountered good ventilation
be provided. The commercial price of zinc in January
2002 was roughly 40¢/lb ($90 kg). Zinc metal with a purity of
99.9999% is priced at about $5/g.
Zinc is a constituent of many common alloys,including brass, bronze, Babbit metal, andGerman Silver. It is used to make householdutensils, castings, printing plates, buildingmaterials, electrical apparatus, dry-cell batteriesand many zinc salts. It is also used to galvanize sheet iron, bleaching bone glue andas a reducing agent in many organic reactions.
This bluish white metallic element is found in sphalerite ore
that is roasted to give an oxide that is reduced with carbon to
make zinc vapor, which is condensed. Elemental zinc foil was
occasionally used to decolorize old collodion rich in iodine.
The zinc halides were used primarily in collodion emulsions.
(Zn) A metallic element that functions as a nutrient and dietary supplement. It is believed to be necessary for nucleic acid metabolism, protein synthesis, and cell growth. Sources of include zinc acetate, carbonate, chloride, citrate, gluconate, oxide, stearate, and sulfate. The gluconate form is used in lozenges. The sulfate form exists as prisms, needles, or powder. It has a solubility of 1 g in 0.6 ml of water and is found in frozen egg substitutes.
Zinc is another earliest known metal. Use of its alloy, brass, dates back to prehistoric times. The metal was produced in India in the 13th century by reducing calamine (a silicate mineral of zinc) with wool. Marggraf produced the metal in 1746 by reducing calamine with charcoal. The element took its name from the German word zink meaning “of obscure origin.” Lohneyes first used this name in 1697. Zinc occurs in nature, widely distributed. The principal ores are sphalerite (and wurtzite) known as zinc blende, ZnS; gahnite, ZnAl2O4; calamine; smithsonite, ZnCO3; franklinite, ZnFe2O4; and zincite, ZnO. Abundance in earth’s crust is about 70 mg/kg and average concentration in sea water is about 10 µg/L.
Some important applications of zinc include galvanizing steel; to produce die castings; as a chemical additive in rubber and paints; in dry cells; in making electrodes; and as a reducing agent. Steel is galvanized by a thin coating of zinc to protect it from corrosion. Such galvanized steel is used in buildings, cars, and appliances. High-purity zinc is alloyed with aluminum at varying compositions, along with small amounts of copper and magnesium, to produce die castings. Such die castings are used extensively in automotive, hardware, and electrical industries. Zinc forms numerous alloys including brass, nickel silver, German silver, commercial bronze, soft solder, aluminum solder, and spring brass. The laboratory use of zinc includes preparating hydrogen gas and as a reducing agent in a number of chemical reactions. Zinc salts have numerous uses (See under specific compounds). Zinc is an essential nutrient element required for growth of animals.
zinc is described as an oligo element, trace element, or micro nutrient. Zinc is believed to accelerate wound healing. It is also considered an anti-oxidant, offering protection against uV radiation. It appears to favor the sulfur uptake in sulfurated amino acids and facilitates the incorporation of cysteine, an amino acid, into the skin. It also has a synergistic effect with vitamins A and e. Zinc is a component of more than 70 metal enzymes. It promotes collagen synthesis in the dermis and keratinization of the corneum layer. Zinc is useful for acne treatments because it lowers sebaceous secretion, and is also used in the treatment of psoriasis.
Zinc is widely distributed in nature, constituting 20–200 ppm
of the Earth’s crust.The principal zinc ore is in the form of sulfides, such as
sphalerite and wurtzite (cubic and hexagonal ZnS) and
willemite (Zn2SiO4). To obtain metallic zinc, the zinc ores
that are relatively low in zinc content are concentrated. Zinc
smelting is gradually being replaced by the electrolytic
processes. During smelting there are often large emissions
of zinc, and other heavy metals contained in the zinc ore such
as lead and cadmium, into the air.
zinc: Symbol Zn. A blue-white metallicelement; a.n. 30; r.a.m. 65.38; r.d.7.1; m.p. 419.88°C; b.p. 907°C. It occursin sphalerite (or zinc blende,ZnS), which is found associated withthe lead sulphide, and in smithsonite(ZnCO3). Ores are roasted to give theoxide and this is reduced with carbon(coke) at high temperature, the zincvapour being condensed. Alternatively,the oxide is dissolved in sulphuricacid and the zinc obtained byelectrolysis. There are five stable isotopes(mass numbers 64, 66, 67, 68,and 70) and six radioactive isotopesare known. The metal is used in galvanizingand in a number of alloys(brass, bronze, etc.). Chemically it is areactive metal, combining with oxygenand other nonmetals and reactingwith dilute acids to releasehydrogen. It also dissolves in alkalis to give zincates. Most of its compoundscontain the Zn2+ ion.
Zinc exhibits a valence of +2 in all its compounds. It also is a highly electropositive metal. It replaces less electropositive metals from their aqueous salt solutions or melts. For example, a zinc metal bar put into Cu2+ solution acquires a brown-black crust of copper metal deposited on it. At the same time the blue color of the solution fades. Zinc reduces Cu2+ ions to copper metal. The overall reaction is:
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
This spontaneous reaction was used first in 1830 to make a voltaic cell. The metal is attacked by mineral acids. Reactions with sulfuric and hydrochloric acids produce hydrogen. With nitric acid, no hydrogen is evolved but the pentavalent nitrogen is reduced to nitrogen at lower valence states. Zinc is attacked by moist air at room temperature. Dry air has no action at ambient temperatures but the metal combines with dry oxygen rapidly above 225°C. Zinc reacts with carbon dioxide in the presence of moisture at ordinary temperatures forming a hydrated basic carbonate. The metal, on heating with dry halogen gases, yields zinc halides. However, in the presence of moisture the reaction occurs rapidly at ambient temperatures. The metal dissolves in hot solutions of caustic alkalis to form zincates and evolves hydrogen:
Zn + 2NaOH → Na2ZnO2 + H2
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
This spontaneous reaction was used first in 1830 to make a voltaic cell. The metal is attacked by mineral acids. Reactions with sulfuric and hydrochloric acids produce hydrogen. With nitric acid, no hydrogen is evolved but the pentavalent nitrogen is reduced to nitrogen at lower valence states. Zinc is attacked by moist air at room temperature. Dry air has no action at ambient temperatures but the metal combines with dry oxygen rapidly above 225°C. Zinc reacts with carbon dioxide in the presence of moisture at ordinary temperatures forming a hydrated basic carbonate. The metal, on heating with dry halogen gases, yields zinc halides. However, in the presence of moisture the reaction occurs rapidly at ambient temperatures. The metal dissolves in hot solutions of caustic alkalis to form zincates and evolves hydrogen:
Zn + 2NaOH → Na2ZnO2 + H2
A grayish powder. Insoluble in water. May produce toxic zinc oxide fumes when heated to very high temperatures or when burned. Used in paints, bleaches and to make other chemicals.
Can evolve gaseous hydrogen in contact with water or damp air. The heat of the reaction may be sufficient to ignite the hydrogen produced [Haz. Chem. Data 1966. p. 171]. Flammable. May form an explosive mixture with air [Hawley].
ZINC METAL is a reducing agent. Reacts violently with oxidants causing fire and explosion hazards [Handling Chemicals Safely 1980. p. 966]. In the presence of carbon, the combination of chlorine trifluoride with zinc results in a violent reaction [Mellor 2, Supp. 1: 1956]. Sodium peroxide oxidizes zinc with incandescence [Mellor 2:490-93 1946-47]. Zinc powder or dust in contact with acids forms hydrogen. The heat generated by the reaction is sufficient to ignite the hydrogen evolved [Lab. Govt. Chemist 1965]. A mixture of powdered zinc and an oxidizing agent such as potassium chlorate or powdered sulfur can be exploded by percussion. Zinc burns in moist chlorine. A mixture of zinc and carbon disulfide reacts with incandescence. Zinc powder reacts explosively when heated with manganese chloride. The reaction between zinc and selenium or tellurium is accompanied by incandescence [Mellor 4:476-480 1946-47]. When zinc and ammonium nitrate are mixed and wetted with a minimum of water, a violent reaction occurs with evolution of steam and zinc oxide. When hydrazine mononitrate is heated in contact with zinc a flaming decomposition occurs at temperatures a little above its melting point. Hydroxylamine is reduced when heated with ZINC, unpredictably ZINC may either ignite and burn or explode [Mellor 8 1946-47].
As mentioned, zinc dust and powder are very explosive. When zinc shavings are placedin acid or strong alkaline solutions, hydrogen gas is produced, which may explode. Many ofzinc’s compounds are toxic if inhaled or ingested.
A deficiency of zinc in humans will retard growth, both physically and mentally, andcontribute to anemia. It is present in many foods, particularly proteins (meat). A balanceddiet provides an adequate amount of zinc. Not more than 50 milligrams per day of dietaryzinc supplement should be taken, given that high levels of zinc in the body are toxic. Humanbodies contain about two grams of zinc. A deficiency of zinc can cause a lack of taste and candelay growth as well as cause retardation in children.
Zinc intoxication can occur both from inhaling zinc fumes and particles, mainly in industrialprocesses, and from orally ingesting an excess of zinc in dietary supplements. Zinc intoxicationcan cause stomach pains, vomiting, and bleeding. Excess zinc also can cause prematurebirth in pregnant women.
A deficiency of zinc in humans will retard growth, both physically and mentally, andcontribute to anemia. It is present in many foods, particularly proteins (meat). A balanceddiet provides an adequate amount of zinc. Not more than 50 milligrams per day of dietaryzinc supplement should be taken, given that high levels of zinc in the body are toxic. Humanbodies contain about two grams of zinc. A deficiency of zinc can cause a lack of taste and candelay growth as well as cause retardation in children.
Zinc intoxication can occur both from inhaling zinc fumes and particles, mainly in industrialprocesses, and from orally ingesting an excess of zinc in dietary supplements. Zinc intoxicationcan cause stomach pains, vomiting, and bleeding. Excess zinc also can cause prematurebirth in pregnant women.
Zinc and its compounds are relatively non-toxic, but very large doses can produce an acute gastroenteritis characterized by nausea, vomiting, and diarrhea. The recommended dietary allowance (RDA) for zinc is 15 mg/day for men, 12 mg/day for women, 10 mg/day for children, and 5 mg/day for infants. Insuffi cient zinc in the diet can result in a loss of appetite, a decreased sense of taste and smell, slow wound healing and skin sores, or a damaged immune system. Pregnant women with low zinc intake have babies with growth retardation. Exposure to zinc in excess, however, can also be damaging to health. Harmful health effects generally begin at levels from 10–15 times the RDA (in the 100–250 mg/day range). Eating large amounts of zinc, even for a short time, can cause stomach cramps, nausea, and vomiting. Chronic exposures to zinc chloride fumes cause irritation, pulmonary edema, bronchopneumonia, pulmonary fi brosis, and cyanosis. It also causes anemia, pancreas damage, and lower levels of high-density lipoprotein cholesterol. Breathing large amounts of zinc (as dust or fumes) can cause a specifi c short-term disease, called metal fume fever, including disturbances in the adrenal secretion. Information on the possible toxicological effects following prolonged period of exposures to high concentrations of zinc is not known.
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.
Zinc (Zn) is a bluish-white metal belonging to the 12th Group of the Periodic Table. It occurs naturally as sphalerite, smithsonite, hemimorphite and wurzite, and is extracted by roasting the oxide and reducing with carbon. It is used for galvanizing,
The average human body contains around 2 g of Zn2+. Therefore, zinc (after iron) is the second most abundant
d-block metal in the human body. Zinc occurs in the human body as Zn2+ (closed d10 shell configuration),
which forms diamagnetic and mainly colourless complexes. In biological systems, zinc ions are often
found as the active centre of enzymes, which can catalyse metabolism or degradation processes, and are
known to be essential for stabilising certain protein structures that are important for a variety of biological
processes.
Already from ancient times, Zn2+ was known to have important biological properties. Zinc-based ointments were traditionally used for wound healing. Low Zn2+ concentrations can lead to a variety of health-related problems especially in connection with biological systems of high Zn2+ demand such as the reproductive system. The daily requirement for Zn2+ is between 3 and 25 mg, depending on the age and circumstances.
The enzymatic function of Zn2+ is based on its Lewis acid activity, which are electron-deficient species. In the following chapters, examples will be shown to further explain this. Carboanhydrase (CA),carboxypeptidase and superoxide dismutase are some examples for well-studied zinc-containing enzymes. The so-called zinc fingers have been discovered because of the crucial role of Zn2+ in the growth of organisms. Within the zinc finger, Zn2+ stabilises the protein structure and therefore enables its biological function.
Already from ancient times, Zn2+ was known to have important biological properties. Zinc-based ointments were traditionally used for wound healing. Low Zn2+ concentrations can lead to a variety of health-related problems especially in connection with biological systems of high Zn2+ demand such as the reproductive system. The daily requirement for Zn2+ is between 3 and 25 mg, depending on the age and circumstances.
The enzymatic function of Zn2+ is based on its Lewis acid activity, which are electron-deficient species. In the following chapters, examples will be shown to further explain this. Carboanhydrase (CA),carboxypeptidase and superoxide dismutase are some examples for well-studied zinc-containing enzymes. The so-called zinc fingers have been discovered because of the crucial role of Zn2+ in the growth of organisms. Within the zinc finger, Zn2+ stabilises the protein structure and therefore enables its biological function.
Hot-dipped or galvanized zinc coatings havebeen popular for many years for protecting ferrousproducts because of their ideal combinationof high corrosion protection and low cost.Their corrosion protection stems from threeimportant factors:zinc has a slower rate ofcorrosion than iron,zinc corrosion productsare white and nonstaining, and zinc affordselectrolytic protection to iron.
The amount of protection against corrosiondepends largely upon coating weight — theheavier the coating, the longer the life of the base metal. For example, a coating 0.04 mmthick is expected to have a life of 25 years inrural atmospheres, whereas a 0.88-mm coatingwill last 50 years. The life of zinc coatings maybe five to ten times greater in rural atmospheresthan in industrial atmospheres containing sulfurand acid gases. Nevertheless, the coatings arestill popular for industrial use because of theirlow cost.Hot dipping is particularly valuable for zinccoating parts that cannot conveniently be madeof galvanized sheet. Thus, it is quite popular forstructural parts, castings, bolts, nuts, nails, polelinehardware, heater and condenser coils,windlasses, and many other products.
The amount of protection against corrosiondepends largely upon coating weight — theheavier the coating, the longer the life of the base metal. For example, a coating 0.04 mmthick is expected to have a life of 25 years inrural atmospheres, whereas a 0.88-mm coatingwill last 50 years. The life of zinc coatings maybe five to ten times greater in rural atmospheresthan in industrial atmospheres containing sulfurand acid gases. Nevertheless, the coatings arestill popular for industrial use because of theirlow cost.Hot dipping is particularly valuable for zinccoating parts that cannot conveniently be madeof galvanized sheet. Thus, it is quite popular forstructural parts, castings, bolts, nuts, nails, polelinehardware, heater and condenser coils,windlasses, and many other products.
Human systemic effects
by ingestion: cough, dyspnea, and sweating.
A human skin irritant. Pure zinc powder,
dust, and fume are relatively nontoxic to
humans by inhalation. The dfficulty arises
from oxidation of zinc fumes immedately
prior to inhalation or presence of impurities
such as Cd, Sb, As, Pb. Inhalation may cause
sweet taste, throat dryness, cough, weakness,
generalized aches, chills, fever, nausea,
vomiting.
Flammable in the form of dust when
exposed to heat or flame. May i p t e
spontaneously in air when dry. Explosive in
the form of dust when reacted with acids.
Incompatible with NH4NO3, BaO2,
Ba(NO3)2, Cd, CS2, chlorates, Cl2, ClF3,
CrO3, (ethyl acetoacetate + tribromoneo-
pentyl alcohol), F2, hydrazine mononitrate,
hydroxylamine, Pb(N3)2, (Mg + Ba(NO3)2 +
BaO2), MnCl2, HNO3, performic acid,
KCLO3, KNO3, K2O2, Se, NaClO3, Na2O2,
S, Te, H2O2 (NH4)2S, As2O3, CS2, CaCl2,
NaOH, chlorinated rubber, catalytic metals,
halocarbons, o-nitroanisole, nitrobenzene,
nonmetals, oxidants, paint primer base,
pentacarbonyliron, transition metal halides,
seleninyl bromide. To fight fire, use special
mixtures of dry chemical. When heated to
decomposition it emits toxic fumes of ZnO.
See also ZINC COMPOUNDS.
Zinc is used most commonly as a
protective coating of other metals. In addition, it is
used in alloys, such as bronze and brass, for electrical
apparatus in many common goods; and in organic
chemical extractions and reductions. Zinc chloride is a
primary ingredient in smoke bombs used by military
for screening purposes, crowd dispersal and occasionally
in firefighting exercises by both military and civilian
communities. In pharmaceuticals, salts of zinc are
used as solubilizing agents in many drugs, including
insulin.
Repeated intratesticular injections
of zinc chloride to chickens and rats have been reported
to produce testicular sarcomas. There is no evidence that zinc
compounds are carcinogenic after administration by any
other route. Zinc oxide, zinc chloride, and zinc stearate
have been classified by the U.S. EPAas belonging to group D.
Zinc enters the air, water, and soil as a result of both natural
processes and human activities. Most zinc enters the environment
as the result of human activities, such as mining, purifying
of zinc, lead, and cadmium ores, steel production, coal
burning, and burning of wastes. These releases can increase zinc
levels in the atmosphere. Waste streams from zinc and other
metal manufacturing and zinc chemical industries, domestic
wastewater, and runoff from soil containing zinc can discharge
zinc into waterways. The level of zinc in soil increases mainly
from disposal of zinc wastes from metal manufacturing
industries and coal ash from electric utilities. In air, zinc is
present mostly as fine dust particles. This dust eventually settles
over land and water. Rain and snow aid in removing zinc from
air. Most of the zinc in bodies of water, such as lakes or rivers,
settles on the bottom. However, a small amount may remain
either dissolved in water or as fine suspended particles. The
level of dissolved zinc in water may increase as the acidity of
water increases. Some fish can collect zinc in their bodies if they
live in water containing zinc. Most of the zinc in soil is bound
to the soil and does not dissolve in water. However, depending
on the characteristics of the soil, some zinc may reach
groundwater. Contamination of groundwater from hazardous
waste sites has been noticed. Zinc may be taken up by animals
eating soil or drinking water containing zinc. If other animals
eat these animals, they will also have increased amounts of zinc
in their bodies.
UN1436 Zinc powder or zinc dust, Hazard
Class: 4.3; Labels: 4.3-Dangerous when wet material,
4.2-Spontaneously combustible material.
Commercial zinc dust (1.2kg) is stirred with 2% HCl (3L) for 1minute, then the acid is removed by filtration, and washed in a 4L beaker with a 3L portion of 2% HCl, three 1L portions of distilled water, two 2L portions of 95% EtOH, and finally with 2L of absolute Et2O. (The wash solutions were removed each time by filtration.) The material is then dried thoroughly, and if necessary, any lumps are broken up in a mortar. [Wagenknecht & Juza Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1067 1965.]
Zinc is essential for humans and animals. It is necessary for
the function of numerous enzymes include alcohol dehydrogenase,
alkaline phosphatase, carbonic anhydrase, and
superoxide dismutase. However, excessive zinc interferes with
iron and copper metabolism; the latter leads to copper-deficiency
anemia. Salts of strong mineral acids are corrosive to
skin and intestine. Zinc also plays an essential role in the
maintenance of the nucleic acid structure of genes and an
integral component of DNA polymerase and RNA polymerase.
Yet, a limited amount of zinc consumed leads to zinc
deficiency. Zinc deficiency decreases the production of DNA
and RNA, which results in the reduction of protein synthesis.
Dust is pyrophoric and may self-ignite in
air. A strong reducing agent. Violent reaction with oxidizers,
chromic anhydride; manganese chloride; chlorates,
chlorine and magnesium. Reacts with water and reacts violently
with acids, alkali hydroxides; and bases forming
highly flammable hydrogen gas. Reacts violently with sulfur,
halogenated hydrocarbons and many other substances,
causing fire and explosion hazard.
Preparation Products And Raw materials
Raw materials
Preparation Products
- DopamineFast Blue BB9,10-DIMETHYLANTHRACENEAzobenzene5-BROMO-2-(METHYLTHIO)PYRIMIDINEChlorotrifluoroethyleneASTRAZON PINK FG1-Amino-4-methylpiperazine2-(4-METHOXYPHENYL)HYDRAZINESULFONIC ACID SODIUM SALT MONOHYDRATE3-Pyrroline4(3H)-Pyrimidinone, 5-methyl-2-(methylthio)-4-(METHYLTHIO)BENZYLAMINE3-AMINO-2-METHYLTHIENO[2,3-D]PYRIMIDIN-4(3H)-ONEZinc bromideChloromethyl silane2,3-DIHYDRO-2-PHENYL-1H-ISOINDOL-1-OXO-ISOINDOLINEN-Methylpropylamine2-(2'-Hydroxy-3',5'-di-tert-butylphenyl)-5-chlorobenzotriazole2-(Phenoxymethyl)benzoic acid2-[(diisopropyl)amino]ethyl 9H-xanthene-9-carboxylate(4-CHLORODIPHENYL)METHYL BETA-CHLOROETHYL ETHER1-Methyl-1-phenylhydrazineAcridineN-HEXADECANE-D345-Methylthianaphtheneround type alkaline zinc-manganese dioxide battery4-(2-AMINO-ETHYL)-2-METHOXY-PHENOL BENZHYDRYL B-CHLOROETHYL ETHER2-METHYL-2-IMIDAZOLINEXANTHENE-9-CARBONITRILEflat type zinc-manganese dioxide battery2-ANTHRACENECARBOXYLIC ACIDZinc iodideZINC PHOSPHIDEPHYSOSTIGMINE2-Aminofluorene4-Chloro-3,5-dimethylphenol4-Ethylpyridineazelaic acid, compound with morpholineWater-soluble inorganic zinc rich primer
Related articles
Zinc CrystalNov 21,2023
Related Product Information
- Arsenic acid,zinc salt
- Bis(dibutyl dithiocarbamato)zinc
- BIS(2,4-PENTANEDIONATO)ZINC HYDRATE
- BENZENESULFINIC ACID ZINC SALT DEHYDRATE,BENZENESULFINIC ACID ZINC SALT DIHYDRATE,BENZENESUIFINIC ACID ZINC SALT
- BIS(2-HYDROXYETHYL)DITHIOCARBAMIC ACID ZINC SALT
- 10-UNDECANOIC ACID ZINC SALT
- FAST SCARLET R SALT
- ZINC RESINATE
- Zinc tetrafluoroborate
- BIS(2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONATO)ZINC(II),BIS(2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONATO)ZINC
1of4
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine