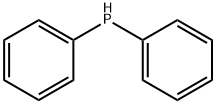
Organophosphorus compound
Diphenylphosphine is commonly used in laboratory as organic phosphorus compound, it is unpleasant odor colorless liquid, and it is pungent, it is easily oxidized in air and can cause spontaneous combustion, it is sensitive to air and light, it need the protection of nitrogen. It can be used as precursor to synthesize some organic phosphine ligand. By deprotonation, it can be converted to diphenylphosphine compound: Ph2PH + nBuLi → Ph2PLi + nBuH, such as 1,2-bis (diphenylphosphino) ethane and 1,3-bis (diphenylphosphino) propane phosphine ligands etc, Wittig-Horner reagents and the synthesis of quaternary phosphonium salt is usually by means of diphenylphosphino alkylation to achieve.
Diphenylphosphine such as sodium and lithium diphenylphosphine and diphenylphosphine compound can add to carbon heteroatom double bond as nucleophile. For example, in the condition of concentrated hydrochloric acid at 100℃, diphenylphosphine can add to aldehyde carbon atoms of benzaldehyde: Ph2PH + PhCHO → Ph2P (O) CH2Ph, when compares with tertiary phosphine, alkalinity of diphenylphosphine is weaker. The pKa of conjugate acid diphenylphosphine is 0.03: Ph2PH2 + → Ph2PH + H +
Preparation: Lithium diphenylphosphine can generated by inexpensive triphenylphosphine and the with the cancellation of water can obtain diphenylphosphine: (1) PPh3 + 2 Li → LiPPh2 + LiPh (2) LiPPh2 + H2O → Ph2PH + LiOH.
The above information is edited by the chemicalbook of Wang Xiaodong.
It can be used the intermediates of organic, catalysts.
Diphenylphosphine is an organophosphorus compound commonly used in laboratories. It is a clear colorless to slightly yellow liquid with unpleasant odor, irritating, easily oxidized in air and spontaneously combusts, sensitive to air and light, and needs to be protected by nitrogen. It can be used as a precursor for the synthesis of a variety of organophosphine ligands. These ligands, in turn, are used in homogeneous catalysis for many applications including: asymmetric hydrogenation, coupling chemistry, ethylene oligomerization, hydroformylation, hydration of nitriles, and polymerization of alkenes.
Diphenylphosphine acts as an intermediate in the preparation of diphenylphosphide derivatives, phosphonium salts, phosphine ligands and Wittig-Horner reagents. The presence of hydrogen atom bonded to phosphorus undergoes Michael-like addition to activated alkenes. It is involved in the preparation of 1,2-bis(diphenylphosphino)ethane and (phenyl-(phenylmethyl)phosphoryl)benzene. Further, it is used in the synthesis of aminophosphines and chiral palladacycles with N-heterocyclic carbene ligands as catalysts.
Diphenylphosphine is used in the synthesis of aminophosphines for application as catalysts. It is also used in the preparation of chiral palladacycles with N-heterocyclic carbene ligands as catalysts.
Diphenylphosphine can be prepared from triphenylphosphine by reduction to lithium diphenylphosphide, which can be protonated to give the title compound:
PPh3 + 2 Li → LiPPh2 + LiPh
LiPPh2 + H2O → Ph2PH + LiOH