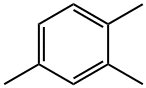
1,2,4-Trimethylbenzene (mesitylene) is an aromatic hydrocarbon compound commonly present in commercial solvents encompassing their boiling range. Mesitylene are the most common isomer used in commercial applications. Aromatic hydrocarbons in hydrocarbon solvents are predominantly alkylated one ring structures as well as two aromatic ring structures which may also be alkylated.
colourless liquid. Insoluble in water, soluble in ethanol, ether and benzene.
Colorless liquid with a slight aromatic odor. A detection odor threshold concentration of 12 mg/m3
(2.4 ppmv) was experimentally determined by Dravnieks (1974).
1,2,4-Trimethylbenzene is the unlabelled version of 1,2,4-Trimethyl 13C6-benzene (T796173), a labelled aromatic standard.
Sterilizing catgut by heating one hour at 160°; solvent in manufacture of dyes, perfumes and resins. Solvent for liquid scintillation counting solutions.
ChEBI: 1,2,4-trimethylbenzene is a trimethylbenzene carrying methyl groups at positions 1, 2 and 4. It has a role as a neurotoxin.
A liquid. Flash point near 130°F. Less dense than water and insoluble in water. Vapors irritate eyes, throat, and nose. Used in dyes and pharmaceuticals.
1,2,4-Trimethylbenzene is incompatible with the following: Oxidizers, nitric acid .
Moderate fire risk. Central nervous system
depressant, irritant to mucous membranes. Asthma
and hematologic effects.
Harmful if inhaled or swallowed. Vapor or mist is irritating to the eyes, mucous membrane and upper respiratory tract. Prolonged contact can cause dermatitis, nausea, headache, dizziness, and narcotic effect.
Moderately toxic by
intraperitoneal route. Mildly toxic by
inhalation. Can cause central nervous system
depression, anemia, bronchitis. Flammable
liquid when exposed to heat, sparks, or
flame. To fight fire, use foam, alcohol foam,
mist. Emitted from modern building
materials (CENEAR 69,22,91). When
heated to decomposition it emits acrid
smoke and irritating fumes.
Acute toxicity studies (oral, dermal and inhalation routes of exposure) have been conducted in rats. Oral LD50 values of greater than 5000 mg/kg have been reported. For solvent products containing predominantly mixed C9 aromatic hydrocarbons, oral LD50 values have been reported as greater than 6880 mg/kg (males) and greater than 3440 mg/kg (females). LC50 values of 18,000 mg/m3 was reported for 1,2,4-TMB. For C9 aromatic solvents, the LC50 was greater than 10,200 mg/m3 (Shell Research Laboratory, 1976, unpublished study). One dermal acute study showed an LD50 value of greater than 3440 mg/kg for a C9 aromatic solvent (Shellsol A, a highly aromatic solvent boiling in the white spirit range, consisting of primarily C9 isomers particularly TMBs)
Biological. In anoxic groundwater near Bemidji, MI, 1,2,4-trimethylbenzene anaerobically
biodegraded to the intermediate 3,4-dimethylbenzoic acid and the tentatively identified
compounds 2,4- and/or 2,5-dimethylbenzoic acid (Cozzarelli et al., 1990).
Photolytic. Glyoxal, methylglyoxal, and biacetyl were produced from the photooxidation of
1,2,4-trimethylbenzene by OH radicals in air at 25 °C (Tuazon et al., 1986a). A rate constant of
2.0 x 10-8 L/molecule?sec was reported for the reaction of 1,2,4-trimethylbenzene with OH radicals
in the gas phase (Darnall et al., 1976). Similarly, the rate constants for the reaction of 1,2,4-
trimethylbenzene and OH radicals at room temperature were 3.35 x 10-11 (Hansen et al., 1975) and
3.84 x 10-11 cm3/molecule?sec (Atkinson, 1985). At 25 °C, a rate constant of 3.15 x 10-11
cm3/molecule?sec was reported for the same reaction (Ohta and Ohyama, 1985). Products
identified from the OH radical-initiated reaction of 1,2,4-trimethylbenzene in the presence of
nitrogen dioxide were 3-hexene-2,5-dione and 2,3-butanedione (Bethel et al., 2000).
Chemical/Physical. 1,2,4-Trimethylbenzene will not hydrolyze in water (Kollig, 1993).
Reflux pseudocumene over sodium and distil it under reduced pressure. [Beilstein 6 H 1088, 6 I 542, 6 II 1072, 6 III 6278, 6 IV 7339.]