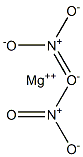The anhydrous salt consists of white cubic crystals; density 2.3 g/cm3; very soluble in water. The dihydrate is white crystalline solid having density 1.45 g/cm3; decomposes at about 100°C; soluble in water and ethanol. The hexahydrate, MgNO3•6H2O is a colorless solid having monoclinic crystal structure and density 1.46 g/cm3. The salt is hygroscopic and very soluble in water and moderately soluble in ethanol.
The hexahydrate, Mg(NO3)2•6H2O, occurs in nature as mineral nitromagnesite. Magnesium nitrate is used in pyrotechnics; and in the manufacture of concentrated nitric acid to remove water and concentrate the acid vapors to 90–95% HNO3. It also is used to aid coating and prilling in production of ammonium nitrate. The salt also is used as an analytical standard for magnesium and a matrix modifier in furnace atomic absorption spectroscopic analysis. It also finds some limited application as a nitrogenous fertilizer.
Magnesium nitrate is prepared by the action of nitric acid on magnesium carbonate, oxide or hydroxide:
MgCO3 + 2HNO3 → Mg(NO3)2 + CO2 + H2O
Mg(OH)2 + 2HNO3 → Mg(NO3)2 + 2H2O
The salt crystallizing at room temperature after evaporation is the hexahydrate, Mg(NO3)2•2H2O.
Thermal decomposition of anhydrous Mg(NO3)2 yields magnesium oxide and nitrogen oxides. Heating the hexahydrate above its melting point forms basic nitrates, such as Mg(NO3)2•4 Mg(OH)2. The latter decomposes at 400°C, forming magnesium oxide and oxides of nitrogen. Magnesium nitrate forms addition compounds with a number of nitrogen-containing organics such as pyridine, aniline, and urea.
Magnesium nitrate has the molecular formula of
Mg(NO3)2 and the molecular weight of 148.3152 g/
mol.
Magnesium nitrate is prepared by the action of nitric
acid on magnesium carbonate, oxide or hydroxide:
MgCO3 + 2HNO3 ? Mg(NO3)2 + CO2 +H2O
Mg(OH)2 + 2HNO3 ? Mg(NO3)2 + 2H2O
The salt crystallizing at room temperature after
evaporation is the hexahydrate, Mg(NO3)2·6H2O. Two
stable hydrates are formed, the hexahydrate [CAS
number =13446-18-9] and the dihydrate, Mg(NO3)2·
2H2O [CAS number = 15750-45-5].
Magnesium nitrate is white crystalline solid.
White crystals.Soluble in water and alcohol;
deliquescent.
It is used in printing, chemical, agriculture and ceramics industries. Its fertilizer grade has 10.5% nitrogen and 9.4% magnesium.
In pyrotechnics; in the concentration of nitric acid.
ChEBI: The inorganic nitrate salt of magnesium.
The magnesium nitrate used in commerce has been synthesized in a variety of ways. The reaction between nitric acid and magnesium metal is one way and reaction with MgO is another. Magnesium hydroxide and ammonium nitrate also form the product but ammonia is released as a by-product:
2HNO3 + Mg Mg(NO3)2 +H2
2HNO3 + MgO Mg(NO3)2 +H2O
Mg(OH)2 + 2NH4NO3 Mg(NO3)2 + 2NH3 + 2H2O
Since magnesium nitrate has a high affinity for water, heating the hexahydrate does not result in the dehydration of the salt. Instead, it decomposes into magnesium oxide, oxygen and nitrogen oxides:
4Mg(NO3)2·6H2O + heat 4MgO + 2NO2 + 2N2O + O2 + 6H2O
Heating the hexahydrate above its melting point first forms basic nitrates, such as Mg(NO3)2·4Mg(OH)2. It is this salt that decomposes at 400 C, forming magnesium oxide and oxides of nitrogen. The absorption of these nitrogen oxides in water is one possible way to synthesize HNO3. Although it is inefficient, it does not require the use of another strong acid and the mineral, nitromagnesite, can be used in this context.
A white crystalline solid. Produces toxic oxides of nitrogen if heated to decomposition. Used in pyrotechnics.
Deliquescent. Water soluble.
Mixtures of Magnesium nitrate with alkyl esters may explode owing to the formation of alkyl nitrates; mixtures with phosphorus, tin(II) chloride, or other reducing agents may react explosively [Bretherick 1979 p. 108-109]. Noncombustible but will accelerate the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided an explosion may result. Prolonged exposure to fire or heat may result in an explosion. Magnesium nitrate has been reported to undergo spontaneous decomposition in dimethylformamide [Bretherick 5th ed., 1995]. Magnesium nitrate tends to behave as a strong oxidizer.
Dangerous fire and explosion risk in contact
with organic materials, strong oxidizing agent.
Exposure can cause mild irritation to the mucous membranes. Symptoms may include coughing and shortness of breath. Ingestion of large doses may cause dizziness, abdominal pain, vomiting, bloody diarrhea, weakness, convulsions, and collapse. Contact with skin may cause irritation, redness, and pain.
Flammability and Explosibility
Non flammable
Probably a severe
irritant to the eyes, skin, and mucous
membranes. A powerful oxidizer. Violent
decomposition on contact with
dmethylformamide. When heated to
decomposition it emits toxic fumes of NOx.
See also NITRATES and MAGNESIUM
COMPOUNDS.
Magnesium nitrate is used in fireworks and in the production of concentrated nitric acid.
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Note to physician: Treat for methemoglobinemia.Spectrophotometry may be required for precise determination of levels of methemoglobin in urine.
Color Code—Yellow: Reactive Hazard (strongoxidizer); Store in a location separate from other materials,especially flammables and combustibles. Prior to workingwith this chemical you should be trained on its proper handling and storage. Magnesium nitrate must be stored toavoid contact with dimethyl formamide, fuels, and strongreducing agents, since violent reactions occur. Store intightly closed containers in a cool, well-ventilated areaaway from flammable and combustible materials. Avoidstorage on wood floors. See OSHA Standard 1910.104 andNFPA 43A Code for the Storage of Liquid and SolidOxidizers for detailed handling and storage regulations.
UN1474 Magnesium nitrate, Hazard Class: 5.1;
Labels: 5.1-Oxidizer
A powerful oxidizer. Violent reaction
with dimethylformamide, reducing agents; combustibles,
fuels, organic and easily oxidizable matter