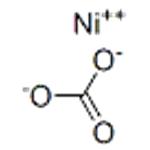
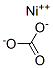Nickel carbonate is a light green crystalline substance, which is almost insoluble (0.093 g/L) in water (25°C), nonsoluble in hot water, and soluble in acids. Nickel carbonate is available primarily as basic nickel carbonate (NiCO3· 2Ni(OH)2 · 4H2O), which is not soluble in water and soluble in ammonia and dilute acids. In the natural environment, nickel carbonate tetrahydrate can be found as zaratite.

NiCO3: Light green rhombohedral crystals; decomposes on heating; practically insoluble in water, 93 mg/L at 25°C; dissolves in acids.
2NiCO3?3Ni(OH)2?4H2O: Light green crystals or brown powder; decomposes on heating; insoluble in water; decomposes in hot water; soluble in acids and in ammonium salts solutions.
Zaratite: Emerald greed cubic crystals; density 2.6 g/cm3; insoluble in water; soluble in ammonia and dilute acids.
Nickel carbonate is used to prepare nickel catalysts and several specialty
compounds of nickel. It also is used as a neutralizing agent in nickel plating
solutions. Other applications are in coloring glass and in the manufacture of
ceramic pigments.
Nickel(II) carbonate is used in sulphamate baths, metal phosphating, electroplating and ceramic applications. It acts as a precursor to catalysts and an intermediate in the hydrometallurgical purification of nickel from its ores.
Anhydrous nickel carbonate is produced as a precipitate when calcium carbonate is heated with a solution of nickel chloride in a sealed tube at 150°C. Alternatively, treating nickel powder with ammonia and carbon dioxide followed by boiling off ammonia yields pure carbonate.
When sodium carbonate is added to a solution of Ni(II) salts, basic nickel carbonate precipitates out in impure form.
Nickel carbonate is the starting material for preparing many nickel salts. It reacts with dilute acids evolving carbon dioxide, and upon evaporation of the solution corresponding nickel salts are formed. The nitrate, sulfate and phosphate salts are prepared from carbonate. Similarly, reactions with hydrofluoric, hydrochloric, hydrobromic, or hydriodic acids yield hydrated nickel halides: namely NiF2•4H2O, NiCl2•6H2O, NiBr2•6H2O, and NiI2•6H2O, respectively:
NiCO3 + HCl → NiCl2•6H2O + CO2
NICKEL CARBONATE 611Nickel carbonate decomposes to nickel oxide when strongly ignited:
NiCO3 → NiO + CO2
Nickel carbonate, when dissolved in aqueous thiocyanic acid, yields a yellow brown precipitate of hydrated nickel thiocyanate:
2 NiCO3 + 2HSCN → Ni(SCN)2 + CO2 + H2O
Nickel carbonate forms many double salts, such as, Na2CO3•NiCO3•10H2O with alkali metal carbonates. However, such double carbonates usually are prepared by mixing an alkali metal or ammonium bicarbonate solution with a nickel salt solution, followed by crystallization.
Confirmed human
carcinogen. When heated to decomposition
it emits toxic vapors of nickel.