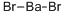Barium bromide has the molecular formula of BaBr2.
Like barium chloride, it is soluble in water but is toxic if
ingested. BaBr2 crystallizes in a PbCl2 motif, as white
orthorhombic crystals that are deliquescent. In aqueous
solution, BaBr2 behaves as a simple salt.
Barium bromide can be crystallized from its solution
as a dihydrate, BaBr2·2H2O (molecular weight: 333.19),
which forms the anhydrate by heating to 120°C. It
dissolves in its own water-of-hydration at 75°C before
decomposing to the anhydrate.
White Crysatalline Powder
White orthorhombic crystal; density of anhydrous BaCl2 4.78 g/cm3, and dihydrate BaCl2 ? 2H2O 3.58 g/cm3; melts at 857°C; vaporizes at 1,835°C; readily dissolves in water (92.2 g/100 g water at 0°C).
In the manufacture of other bromides; in the preparation of phosphors.
Barium bromide is
a precursor to chemicals used in photography as well
as to form other bromides.Historically, barium bromide was used to purify
radium in a process of fractional crystallization devised
by Marie Curie. Since radium precipitates preferentially
in a solution of barium bromide, the ratio of radium to
barium in the precipitate was found to be higher than
the ratio in the solution.
Barium bromide, anhydrous is used as the chemical intermediate, and the spice cosmetics chemical additive. It is also used as pharmaceutical intermediate.
Barium bromide can be prepared from barium
sulfide or barium carbonate by reaction with hydrobromic
acid to give hydrated barium bromide:
BaS+HBr→BaBr2+H2S
BaCO3+HBr→BaBr2+CO2+H2O
Ingestion of the salt or its aqueous solution can produce severe poisoning.