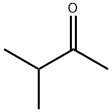
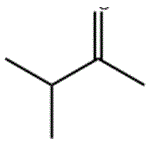
MIPK is a colorless liquid with an acetonelike odor. Molecular weight = 86.15; Specific gravity(H2O:1) 5 0.81; Boiling point = 93℃. Freezingpoint 5 2 92℃; Vapor pressure 5 42 mmHg at 20℃; Flashpoint 5 6℃; Autoignition temperature 5 475℃. Explosivelimits: LEL 5 1.2%; UEL 5 8%. Hazard Identification(based on NFPA-704 M Rating System): Health 1,Flammability 4, Reactivity 0. Slightly soluble in water;solubility 5 0.5% at 20℃.
MIPK is a colorless liquid with an acetonelike odor. 3-Methyl-2-butanone is a colorless liquid with a characteristic ketone odor.3-Methyl-2-butanone undergoes condensation reactions preferentially at the methyl group. The tertiary carbon atom is easily oxidized and the resulting hydroperoxide (mp 26 – 27℃) can be produced by base-catalyzed air oxidation.
Methyl isopropyl ketone (MIPK) is used asa solvent for lacquers.
3-Methyl-2-butanone is used for synthesis of heterocyclic compounds and important intermediate of medicine. It is used as an industrial solvent.
Solvent for nitrocellulose lacquers. 3-Methyl-2-butanone is employed predominantly as an intermediate for the production of pharmaceuticals, herbicides, and dye precursors. It is also used in the synthesis of rubber auxiliaries and for the selective extraction of rare earth elements.
ChEBI: 3-Methylbutan-2-one is a ketone.
A colorless liquid with a pleasant odor. Flash point below 70°F. Less dense than water. May be toxic by inhalation and skin absorption. Used as a solvent.
Highly flammable. Soluble in water.
Ketones, such as 3-Methyl-2-butanone, are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Toxic material. Embryo/fetal damage andneonatal toxicity.
The acute toxic effects from exposure to thevapors of MIPK are narcosis and irritationof the eyes, skin, and respiratory passages.In rabbits the irritation effect of 100 mg fora 24-hour exposure was mild in the eyes,while 500 mg was moderate on the skin. Thetoxicity data on this compound are too scant.In general, its toxicity is low and narcosissymptoms in humans may be felt only at veryhigh concentrations. In guinea pigs exposureto 5000 ppm caused coma.
Irritating to the eyes, nose, throat, upper respiratory tract, and skin.
Special Hazards of Combustion Products: Acrid smoke and fumes
Flammability and Explosibility
Highly flammable
Poison by ingestion.
Mildly toxic by inhalation and skin contact.
Mutation data reported. A skin and eye
irritant. Flammable when exposed to heat or
flame; can react vigorously with oxidizing
materials. When heated to decomposition it
emits acrid smoke and irritating fumes.
This ketone is used as a solvent for
nitrocellulose lacquers.
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist. Store intightly closed containers in a cool, well-ventilated areaaway from aldehydes. Sources of ignition, such as smokingand open flames, are prohibited where methyl isopropylketone is used, handled, or stored in a manner that couldcreate a potential fire or explosion hazard. Use only nonsparking tools and equipment, especially when opening andclosing containers of methyl isopropyl ketone. Wherevermethyl isopropyl ketone is used, handled, manufactured, orstored, use explosion-proof electrical equipment andfittings.
UN23973-Methylbutan-2-one, Hazard Class: 3;
Labels: 3-Flammable liquid.
Reflux the ketone with a little KMnO4. Fractionate it through a spinning-band column, dry with CaSO4 and distil it. [Beilstein 1 IV 3287.]
Vapors may form explosive mixture with
air. Ketones are incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides, nitrated amines, azo,
diazo, azido compounds, carbamates, organic cyanates
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All
federal, state, and local environmental regulations must
be observed.