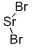Strontium bromide has the formula SrBr2. At room
temperature, it is a white, odorless, crystalline powder
with the molecular weight of 247.43 g/mol. Strontium
bromide burns bright red in a flame test. It is used in
flares and also has some pharmaceutical uses. Several
hydrates have been characterized including the monohydrate
and the hexahydrate, SrBr2·6H2O. This salt is
a white, hygroscopic powder soluble in water and
alcohol. It loses water at 180°C, melts at 643°C and is
used in medicine and as an analytical reagent.
The anhydrate, SrBr2, is a colorless, transparent,
crystal, odorless, having a bitter, saline taste. It is very
deliquescent and soluble in water and is readily soluble
in alcohol and amyl alcohol. The specific gravity of SrBr2
is 4.210 g/cm3. It can be prepared by treating SrCO3 with
a hydrogen bromide solution. Alternatively, a strontium salt (carbonate, sulfate, chloride, etc.) is treated with bromine or hydrobromic acid in presence of a reducing agent.