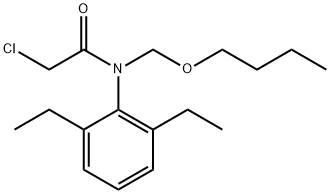
Machette [also known as butachlor] belongs to the chloroacetanilide group herbicides. Butachlor, together with other chloroacetanilide-class herbicides including acetochlor, alachlor, metachlor, and propachlor, are the most consumed chemicals all over the world in agriculture. Butachlor [N-[butoxymethyl]-2-chloro-2’,6’-diethyl acetanilide] is a widely recommended herbicide for application in rice cultivation. It is a systemic selective pre-emergent herbicide applied on rice, tea, wheat, beans and other crops viz. corn, soybean[1]. Butachlor is most commonly used to control a wide range of annual grass and broad leaf weeds[2] as well as submerged macrophytes in freshwater fishponds. Butachlor was developed by Monsanto Co. [USA] in 1968 and commonly used as a post-emergence herbicide in Asia and Africa[3]. It can be manufactured by the reaction of chloroacetyl chloride with the azomethine of 2,6-diethylaniline and formaldehyde, followed by treatment with n-butanol. Butachlor was introduced to Japan in 1973 for weed control in transplanted rice paddies.
Butachlor is a chloroacetanilide herbicide, which is used extensively all over the world as pre-emergence control of unwanted weeds. It is used for controlling a wide range of annual grass and broad leaf weeds[2] as well as submerged macrophytes in freshwater fishponds.
The mode of action of butachlor is by inhibiting the elongase responsible for the elongation of very long-chain fatty acids and the geranylgeranyl pyrophosphate cyclization enzymes[4]. It also affects the various other metabolic processes and redox homeostasis adversely, in addition to lipid biosynthesis[5].
Butachlor can cause severe toxicity.
Acute toxicity
Butachlor causes slight erythema and edema in rabbits when exposed to 24 h continuously[6]. It can also cause primary ocular irritation in 2 of 6 white rabbits tested. It can also cause dermal sensation, moderate-to-severe erythema with edema in guinea pig, where the dermal hypersensitivity was checked in a modified Buehler assay.
Human and Animal toxicity
Butachlor can cause DNA strand breaks and chromosomal aberrations in mammalian cells exhibited[7]. Butachlor can also trigger necrosis in human PBMN cells due to their oxidative role in intracellular reactive oxygen species[ROS] production, and the consequent mitochondrial dysfunction, oxidative DNA damage and chromosomal breakage[8]. Butachlor has mutagenic effects in primary rat tracheal epithelial cells and in Chinese hamster ovarian cells[9]. It is also known cause stomach tumors in rats[10].
- Dwivedi S, Saquib Q, Al-Khedhairy AA, Musarrat J[2012] Butachlor induced dissipation of mitochondrial membrane potential, oxidative DNA damage and necrosis in human peripheral blood mononuclear cells. Toxicology 302:77–87
- Wang S, Li H, Lin C[2013] Physiological, biochemical and growth responses of Italian ryegrass to butachlor exposure. Pestic Biochem Phys 106:21–27
- Liu W-Y, Wang C-Y, Wang T-S, Fellers GM, Lai B-C, Kam Y-C[2011] Impacts of the herbicide butachlor on the larvae of a paddy field breeding frog[Fejervarya limnocharis] in subtropical Taiwan. Ecotoxicology 20:377–384
- Go¨tz T, Bo¨ger P[2004] The very-long-chain fatty acid synthase is inhibited by chloroacetamides. Z Naturforsch C 59:549–553
- Agrawal C, Sen S, Singh S, Rai S, Singh PK, Singh VK, Rai LC[2014] Comparative proteomics reveals association of early accumulated proteins in conferring butachlor tolerance in three N2-fixing Anabaena spp. J Proteomics 96:271–290
- Wilson AGE, Takei AS[2000] Summary of toxicology studies with butachlor. J Pestici Sci 25[1]:75–83
- Panneerselvam N, Sinha S, Shanmugam G[1999] Butachlor is cytotoxic and clastogenic and induces apoptosis in mammalian cells. Indian J Exp Biol 37:888–892
- Dwivedi S, Saquib Q, Al-Khedhairy AA, Musarrat J[2012] Butachlor induced dissipation of mitochondrial membrane potential, oxidative DNA damage and necrosis in human peripheral blood mononuclear cells. Toxicology 302:77–87
- Hill AB, Jefferies PR, Quistad GB, Casida JE[1997] Dialkylquinoneimine metabolites of chloroacetanilide herbicides induce sister chromatid exchanges in cultured human lymphocytes. Mutat Res 395:159–171
- Xu X, Yang H, Wang L, Han B, Wang X, Lee FS-C[2007a] Analysis of chloroacetanilide herbicides in water samples by solid-phase microextraction coupled with gas chromatography–mass spectrometry. Anal Chim Acta 591:87–96
Butachlor is a pre-emergent chloroacetanalide herbicide. Butachlor is commonly used for weed control in rice as well as cotton, maize, wheat and other crops.
Pre-emergence selective anilide herbicide used
to control grass and broad-leaved weeds in seeded
and transplanted rice paddies.
ChEBI: Butachlor is an aromatic amide that is 2-choro-N-(2,6-diethylphenyl)acetamide in which the amide nitrogen has been replaced by a butoxymethyl group. It has a role as a herbicide, an environmental contaminant and a xenobiotic. It is an aromatic amide, an organochlorine compound and a tertiary carboxamide. It is functionally related to a N-phenylacetamide.
Herbicide: Used for pre-emergence control of annual grasses,
sedges and broadleaf weeds in rice crops. Used primarily
in Asia, South America, Europe and Africa. Not registered
for use in the U.S. Not approved for use in EU countries.
BUTANEX®; BUTANOX®; CP 53619®;
HILTACHLOR®; LAMBAST®; MACHETE®;
MACHETTE®; PILLARSET®; RASAYANCHLOR®;
WEEDOUT®; VENDAVAL®
In vitro incubation of butachlor with rat liver fractions
forms a considerable amount of glutathione conjugate,
while the conjugating activity is not efficient for the
kidney S9 fraction. Further biotransformation of the
glutathione conjugate to mercapturate is not observed
in the liver S9 fraction. Butachlor is initially conjugated
with glutathione in the liver and is apparently
transported to the kidneys where it is transformed to
mercapturic acid.