2,4-Dinitroaniline is a nitro substituted benzamide derivative with anti-inflammatory activities in vitro.
2,4-Dinitrochlorobenzene is aminated with aqueous ammonia initially at 70 ℃; then the temperature is allowed to rise gradually to 120 ℃ to complete the reaction. Only a low-pressure autoclave is required, compared with the amination of 2- chloro- and 4- chloronitrobenzene.
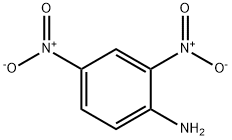
ChEBI: 2,4-dinitroaniline is a nitroaniline consisting of an aniline core having two nitro substituents located at the 2- and 4-positions.
Yellow powder or crystals with a musty odor. Sinks in water.
2,4-Dinitroaniline may decompose violently at elevated temperatures. 2,4-Dinitroaniline can react with oxidizing materials, i.e. chlorine/hydrochloric acid. . In mixture with powdered charcoal ignited upon heating, [Cahiers, 1980, (99), 278].
May cause headache, nausea, stupor. Irritating to skin and mucous membrane.
Agricultural Chemical; Mutagen; Reproductive Effector; Primary Irritant. Used as a corrosion inhibitor, as a dye or pigment intermediate, as a toner pigment in printing inks.
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. In the case of poisoning, special first aid is required; antidotes for the formation of methemoglobin should be available, including instructions. Note to physician: Treat for methemoglobinemia. Spectrophotometry may be required for precise determination of levels of methemoglobinemia in urine.
(1) Color Code—Yellow Stripe: Reactivity Hazard; Store separately in an area isolated from flammables, com bustibles, or other yellow-coded materials. (2) Color Code— Blue: Health Hazard/Poison: Store in a secure poison loca tion. Prior to working with DNA you should be trained on its proper handling and storage. Before entering confined space where DNA may be present, check to make sure that an explosive concentration does not exist. Store in tightly closed containers in a cool, well-ventilated area away from oxidizers, strong acids, strong bases, and other incompatible materials listed above. Metal containers involving the transfer of this chemical should be grounded and bonded. Where possible, automatically pump liquid from drums or other storage containers to process containers. Drums must be equipped with self-closing valves, pressure vacuum bungs, and flame arrest ers. Use only nonsparking tools and equipment, espe cially when opening and closing containers of this chemical. Sources of ignition, such as smoking and open flames, are prohibited where this chemical is used, handled, or stored in a manner that could create a potential fire or explosion hazard.Wherever this chemical is used, handled, manufactured, or stored, use explosion-proof electrical equipment and fittings.
This compound requires a shipping label of “POISONOUS/TOXIC MATERIALS.” Nitroanilines fall into Hazard Group 6.1 and Packing Group II.
Crystallise the nitroaniline from boiling EtOH by adding one-third volume of H2O and cooling slowly. Dry it on a steam oven. The N-acetyl derivative has m 180o. [Beilstein 12 IV 1689.]
Decomposes in moderate heat forming toxic vapors that form an explosive mixture with air. Violent reaction with strong oxidizers, strong acids, strong bases, acid chlorides, acid anhydrides, and chloroformates.