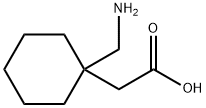
Gabapentin is a second- generation antiepileptic drug (AED) known under the proprietary brand name of Neurontin® (Pfizer, New York, NY) in the UK and USA.
MHRA/ CHM advice to minimize risk when switching patients with epilepsy between different manufacturers’ products (including generic products):
- It is usually unnecessary to ensure that patients are maintained on a specific manufacturer’s product unless there are specific concerns, such as patient anxiety and risk of confusion/ dosing error.
Epilepsy: monotherapy or adjunctive therapy of focal seizures with or without secondary generalization.
Recommendations summarized from NICE (2012)
- Seizure types: adjunctive (focal seizures), contraindicated (generalized tonic- clonic seizures generalized tonic- clonic seizures if there are absence or myoclonic seizures, or if juvenile myoclonic epilepsy is suspected, tonic/ atonic seizures, absence seizures, myoclonic seizures).
- Epilepsy types: adjunctive (benign epilepsy with centrotemporal spikes, Panayiotopoulos syndrome, late- onset childhood occipital epilepsy), contraindicated (absence syndromes, juvenile myoclonic epilepsy, idiopathic generalized epilepsy, Dravet syndrome, Lennox– Gastaut syndrome)
- Psychiatry— treatment of anxiety disorders (unlicensed); treatment of alcohol withdrawal symptoms (unlicensed).
- Neurology— treatment of neuropathic pain and restless leg syndrome (unlicensed) and prophylaxis of migraine (unlicensed).
Monotherapy or adjunctive therapy
300 mg od for day 1300 mg bd for day 2300 mg td for day 3 (or 300 mg td for day 1), then increased by 300 mg every 2– 3 days, divided into three doses; usual maintenance 900– 3600 mg daily, divided into three doses (max. 4800 mg daily) If gabapentin has to be discontinued, it is recommended this should be done gradually over a minimum of 1 week, independent of the indication.
- Patients with a history of psychotic illness.
- Patients with mixed seizures (including absences).
- Patients with diabetes mellitus.
- Elderly patients.
With AEDs
Nil.
With other drugs
- Patients who require concomitant treatment with opioids should be carefully observed for signs of respiratory depression and/ or sedation, and the dose of gabapentin or opioid should be reduced appropriately
- Co-administration of gabapentin with antacids containing aluminium and magnesium, reduces gabapentin bioavailability up to 24%, and it is therefore recommended that gabapentin be taken at the earliest 2 hours following antacid administration
With alcohol/food
There are no known specific interactions between alcohol and gabapentin and there are no specific foods that must be excluded from diet when taking gabapentin.
Renal impairment
Reduce maintenance dose according to degree of reduction in creatinine clearance.
Pregnancy
- The dose of gabapentin should be monitored carefully during pregnancy and after birth, and adjustments made on a clinical basis.
- No definite conclusion can be made as to whether gabapentin is associated with an increased risk of congenital malformations when taken during pregnancy. Gabapentin should not be used during pregnancy unless the potential benefit to the mother clearly outweighs the potential risk to the foetus.
- Gabapentin is excreted in human milk. Because the effect on the breastfed infant is unknown, gabapentin should be used in breastfeeding mothers with caution and only if the benefits clearly outweigh the risks.
Behavioural and cognitive effects in patients with epilepsy
Gabapentin has a relatively favourable behavioural profile, although paradoxical hyperactivity, irritability and aggression have been occasionally reported, especially in patients with severe intellectual disabilities. The cognitive profile of gabapentin is equally favourable, as this AED has been associated with only minor cognitive difficulties (mainly in the attention domain).
Although gabapentin has no approved indications in psychiatry, it has shown efficacy in the treatment of anxiety disorders, especially social phobia. Other offlabel uses include other anxiety disorders (panic disorder, post- traumatic stress disorder), alcohol dependence and withdrawal, behavioural and psychological symptoms of dementia, and aggression. Gabapentin has also been proposed to be useful in the maintenance treatment of bipolar disorder as adjunctive therapy.
γ-Aminobutyric acid (GABA) is a major inhibitory neurotransmitter that functions by binding to the GABA receptors located in the spinal cord. Gabapentin is a γ-aminobutyric acid (GABA) analogue that acts as an anticonvulsant with proven analgesic effects in various neuropathic pain syndromes such as Complex Regional Pain Syndrome type one (CRPS 1). It is also prescribed to multiple sclerosis patients to control dysesthesias and may be useful in reducing neuropathic pain caused by cancer and HIV infection. It does not bind to GABA receptors, does not influence neural uptake of GABA, and does not inhibit the GABA-metabolizing enzyme, GABA transaminase. Unlike GABA, which does not pass through the blood-brain barrier, gabapentin penetrates into the central nervous system and binds to the α2δ-type voltage-gated calcium channels. The mechanism for the analgesic and anticonvulsant effects of gabapentin are not known.
Gabapentin was introduced in 1993 in the UK and early 1994 in the USA as an
adjunctive therapy in the treatment of refractory partial seizures and secondarily generalized
tonic-clonic seizures. Although being a lipophilic analog of the neurotransmitter GABA,
gabapentin appears to exert its anticonvulsive function by a GABA receptor independent
mechanism, possibly involving the L-system amino acid transporter protein. Gabapentin
easily crosses the blood brain barrier and exhibits a favorable pharmacokinetic profile with
high tolerability. It does not interfere with the metabolism of other concomitant administered
antiepileptic drugs, thus having a low potential for drug interactions. Studies are currently
underway for the use of gabapentin as mono-therapy for the treatment of various seizures.
For the treatment of adult Restless Legs Syndrome (RLS) and postherpetic neuralgia (PHN).
antipsychotic, 5HT2A antagonist
Gabapentin is an Amino acid structurally related to γ-Aminobutyric Acid (GABA), designed to cross the blood brain barrier. Used as an anticonvulsant.
selective H1-receptor antagonist
Gabapentin (Neurontin) significantly decreases pain scores and sleep interference
associated with PHN. An initial dose of 300 mg/day is increased
over 4 weeks (900, 1,800, 2,400, 3,600 mg/day divided t.i.d.) until efficacy
is obtained or side effects become intolerable.
ChEBI: A gamma-amino acid that is cyclohexane substituted at position 1 by aminomethyl and carboxymethyl groups. Used for treatment of neuropathic pain and restless legs syndrome.
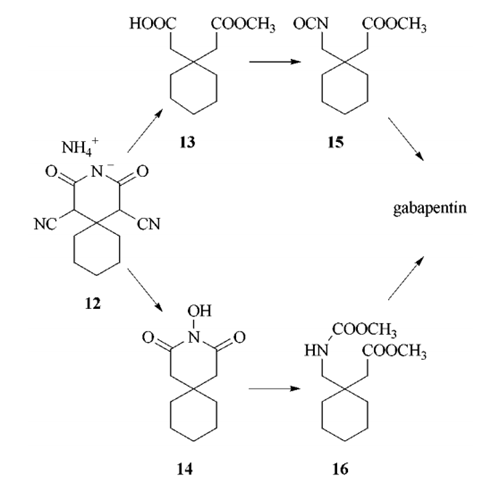
32.8 g 1,1-cyclohexane-diacetic anhydride are mixed with 7 g anhydrous
methanol and heated under reflux for 1 hour. After evaporation of the reaction
mixture in a vacuum, was obtained 37.5 g monomethyl 1,1-cyclohexane�diacetate in the form of a yellowish oil.
5.6 ml triethylamine in 16 ml anhydrous acetone are added dropwise at 0°C to a solution of 7.28 g monomethyl 1,1-cyclohexane-diacetate, then a solution
of 3.6 ml ethyl chloroformate in 16 ml anhydrous acetone is added thereto.
The reaction mixture is further stirred for 30 min at 0°C and and then a
solution of 3.4 g sodium azide in 12 ml water added dropwise thereto. The
reaction mixture is stirred for 1 hour at 0°C, then poured into ice water and
extracted three times with 50 ml amounts of ice-cold toluene. The combined
extracts are dried over anhydrous sodium sulphate at 0°C and subsequently
introduced drop-wise into a flask pre-heated to 100°C. The mixture is then
heated for a further hour under reflux and thereafter evaporated in a vacuum.
The crude methyl 1-isocyanatomethyl-1-cyclohexane-acetate which remains
behind is heated under reflux for 3 hours with 50 ml 20% hydrochloric acid.
After cooling the solution, it is extracted three times with 100 ml amounts of
chloroform to remove the 1-amino-methyl-1-cyclohexane-acetic acid lactam
formed as a by-product product and the aqueous hydrochloric acid solution
evaporated in a vacuum, whereby 1-aminomethyl-1-cyclohexane-acetic acid
crystallises as the hydrochloride; m.p. 117-118°C, after recrystallisation from
acetone/methanol/ether. After recrystallization from methanol/ether the
melting point of the product is 129-133°C.
By treatment with a basic ion exchanger and crystallisation from
ethanol/ether, there is obtained pure 1-amino-methyl-1-cyclohexane-acetic
acid; melting point 162-166°C.
Neurontin (ParkeDavis); Neurontin (Pfizer).
Gabapentin (Neurotonin) was initially designed to be a
rigid analogue of GABA. When it was discovered to
have antiepileptic properties, it was assumed that this
activity was related to a GABAergic mechanism.
However, subsequent studies have failed to show any
GABAergic activity of gabapentin. Although it has not
yet been possible to ascribe any definite mechanism to
its antiepileptic activity, there is recent evidence that it
may function as an agonist at GABAB receptors in the
brain.
Gabapentin is recommended as adjunctive therapy
in the treatment of partial seizures in adults.When used
with other drugs, it appears to be an effective AED; it is
usually not effective when employed alone for patients
with severe seizures.
Gabapentin is generally well tolerated, with somnolence,
dizziness, and ataxia the most commonly reported
adverse effects. A low incidence of potentially serious side effects and no significant allergic reactions have
been reported.
Gabapentin and its closely related analog pregabalin,(S)-3-isobutyl-GABA, are broad-spectrum anticonvulsantswith multiple mechanisms of action.24,51 Inaddition to modulating calcium influx and stimulateGABA biosynthesis as discussed earlier, they also competefor the biosynthesis of L-glutamic acid because oftheir structural similarity to L-leucine.51 Gabapentin andpregabalin have very little liability for causing metabolicbaseddrug–drug interactions, particularly when used incombination with other AEDs because they are not metabolizedin humans. More than 95% of the drug is excretedunchanged through the kidneys. However, there are somedifferences in their bioavailability. Unlike gabapentin,which exhibits 60% bioavailability when given in lowdoses because of intestinal uptake by a saturable smallneutral L-amino acid transporter, the absorption of pregabalinis almost complete (98%) and exhibits an ideal linear pharmacokinetic profile.24 This high bioavailability of pregabalincan be attributed to its closer structure similarity tothe essential amino acid, L-leucine.
Anticonvulsant with several possible mechanisms of action. Increases GABA in the brain and binds to a novel site associated with voltage-sensitive Ca 2+ channels. Prevents neuronal death and is antinociceptive and anxiolytic.
Primary Targetα2δ subunit of L-type voltage gated Ca2+ channels
Antiepileptic:
Adjunctive treatment of partial seizures with or
without secondary generalisation
Neuropathic pain
Migraine prophylaxis (unlicensed)
Dose-limiting adverse effects
include somnolence, dizziness, ataxia, peripheral edema, and infection (22).
Long-Term Side Effects of Gabapentin:
- Mood changes.
- Behavioral changes.
- Depression.
- Anxiety.
- Memory loss.
- Weakened muscles.
In the original synthesis
(Goedecke) cyclohexenone is reacted with
ethyl cyanoacetate in the presence of ammonia
to yield the Guareschi salt, which is hydrolyzed
and decarboxylated to give 1,1-cyclohexanediacetic
acid which is transformed by to
the corresponding anhydride with acetic anhydride.
This anhydride is treated with methanol to
yield the half ester 2-
acetic acid, which is subjected to a Curtius
type rearrangement to give the isocyanate
2-acetic acid.
The desired compound is obtained by hydrolysis
of 2-acetic
acid with HCl, followed by hydrochloric salt removal
via anion exchange .
Veterinary Drugs and Treatments
Gabapentin may be useful as adjunctive therapy for refractory or
complex partial seizures, or in the treatment of chronic pain in dogs
or cats.
Potentially hazardous interactions with other drugs
Antacids: reduce absorption.
Antidepressants: antagonism of anticonvulsive effect
(convulsive threshold lowered); avoid with St John’s
wort.
Antimalarials: anticonvulsant effect antagonised by
mefloquine.
Antipsychotics: antagonism of anticonvulsive effect
(convulsive threshold lowered).
Orlistat: possible increased risk of convulsions.
There is no evidence of gabapentin metabolism in humans.
Gabapentin is eliminated unchanged solely by renal
excretion.
1) Cheng et al. (2004), Does gabapentin act as an agonist at native GABA(B) receptors?; J. Biomed. Sci., 11 346
2) Lanneau et al. (2001), Gabapentin is not a GABAB receptor agonist; Neuropharmacology 41 965
3) Hendrich et al. (2008), Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin; Proc. Natl. Acad. Sci. USA, 105 3628