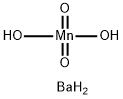
Barium manganate
- Product NameBarium manganate
- CAS7787-35-1
- MFBaH4MnO4
- MW260.29
- EINECS232-109-6
- MOL File7787-35-1.mol
Chemical Properties
| Density | 4.85 g/mL at 25 °C (lit.) |
| form | Fine Crystalline Powder |
| Specific Gravity | 4.85 |
| color | Blue to dark blue-black |
| Water Solubility | Slightly soluble in water |
| Sensitive | Moisture Sensitive |
| Merck | 14,981 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3; TWA 0.1 mg/m3 OSHA: Ceiling 5 mg/m3 NIOSH: IDLH 500 mg/m3; TWA 1 mg/m3; STEL 3 mg/m3 |
| EPA Substance Registry System | Manganic acid (H2MnO4), barium salt (1:1) (7787-35-1) |
Safety Information
| Hazard Codes | Xn,O |
| Risk Statements | 20/22-8 |
| Safety Statements | 28-28A |
| RIDADR | UN 1479 5.1/PG 2 |
| WGK Germany | 1 |
| F | 21 |
| TSCA | Yes |
| HazardClass | 5.1 |
| PackingGroup | III |
| HS Code | 28416900 |