yellow solid with a characteristic odour
1-Chloro-2-nitrobenzene was used in the synthesis of 1-hydroxybenzotriazole derivatives. It is important as a precursor to other compounds due to the two reactive sites present on the molecule.
Chemical intermediate in manufacture
of dyes, picric acid, lumber preservatives, and
diaminophenol hydrochloride (a photographic
developer)
Intermediate, especially for dyes.
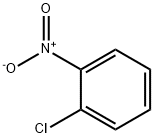
ChEBI: 1-chloro-2-nitrobenzene is a C-nitro compound that is nitrobenzene in which one of the ortho- hydrogens has been replaced by chlorine. It is a C-nitro compound and a member of monochlorobenzenes.
Nitration of chlorobenzene with mixed acid (30/56/14) typically gives an isomer mix in 98 % yield consisting of 34 – 36 % 2-Nitrochlorobenzene, 63 – 65 % 4- chloronitrobenzene, and only ca. 1 % 3- chloronitrobenzene. The ortho – para ratio of about 0.55 is in sharp contrast to the 1.6 obtained with nitrotoluene isomers. As with the nitration of toluene, much work has been done on isomer control so that the producer might have some flexibility towards the balance of isomer demand. Although no major change in ratio has been achieved, the situation is better for nitrochlorobenzene than nitrotoluenes, because the favored isomer is in much greater demand. In further contrast to the nitration of toluene, the nitration of chlorobenzene in the presence of phosphoric acid decreases the proportion of 4-Nitrochlorobenzene, and the use of phosphoric acid in the presence of a transition-metal catalyst is said to increase the ortho – para ratio to ca. 0.8.
Yellow crystals with an aromatic odor. Sinks in water.
2-Nitrochlorobenzene may be sensitive to prolonged exposure to air and light. Insoluble in water.
O-NITROCHLOROBENZENE is incompatible with strong bases and strong oxidizing agents. 2-Nitrochlorobenzene will react with ammonia.
Toxic by inhalation and ingestion. Combustible. Questionable carcinogen.
INHALATION: Headache, languour, anemia. Irritation of nose and throat, cyanosis, shallow respiration, convulsions, and coma. EYES: Irritation. SKIN: Irritation. INGESTION: Forms methemoglobin giving rise to cyanosis and blood changes.
Flammability and Explosibility
Not classified
Suspected carcinogen
with experimental carcinogenic and
neoplastigenic data. Poison by ingestion,
skin contact, and probably inhalation.
Combustible when exposed to heat or
flame. To fight fire, use water, foam.
Potentially explosive reaction with ammonia
at 160℃/30 bar. When heated to
decomposition it emits toxic fumes of Cl-,
NOx, and phosgene. See also other
chloronitrobenzene entries and NITRO COMPOUNDS of AROMATIC
HYDROCARBONS.
Crystallise it from EtOH, MeOH or pentane (charcoal). [Beilstein 5 IV 721.]