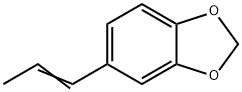
Isosafrole has an anise odor. It may be synthesized by alkaline
isomerization of safrole using KOH at the boil or an alcoholic
NaOH solution at room temperature under pressure.
CLEAR SLIGHTLY YELLOW LIQUID
Isosafrole has an anise-like odor. Use of isosafrole in foods is not permitted in the United States
Of the two isomers (cis- and trans-), the trans-form is the more stable and has been isolated in the pure state,
probably occurring in the essential oil of ylang-ylang; it has been identified in the oils of Illicium religiosum and Ligusticum acuti�lobum Sieb. and Zucc.
Manufacture of heliotropin, perfumes, flavors,
pesticide synergists.
From safrole by treatment with potassium or sodium hydroxide in the dry state or
alcoholic solution, under pressure or at atmospheric pressure (Arctander, 1969).
ChEBI: Isosafrole is a member of benzodioxoles.
Colorless fragrant liquid with odor of anise. Used in small quantities in root beer and sarsaparilla flavors.
ISOSAFROLE may react with strong reducing agents.
On oxidation, isosafrole gives rise initially to an allyl alcohol and another, unidentified, conjugated alcohol, which are further oxidized to the vinyl ketone and piperonyl acrolein, respectively. Condensation of On oxidation, isosafrole gives rise initially to an allyl alcohol and another, unidentified,
conjugated alcohol, which are further oxidized to the vinyl ketone and piperonyl acrolein,
respectively. Condensation of the vinyl ketone with an amine would then lead to the formation
of tertiary aminomethylenedioxypropiophenones (Mannich bases) (McKinney, Oswald, Fishbein &
Walker, 1972).