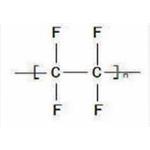
Poly-(Tetrafluoroethylene) (PTFE, Brand name: Teflon, Fluon, Hostaflon, and Polyflon) is a kind of strong, tough, waxy and nonflammable synthetic resin. Its structure formula is [CF2-CF2]n with a carbon backbone chain. It is produced through the free-radical polymerization of tetrafluoroethylene. PTFE has slippery surface, high melting point, and is extremely chemically inert. This property make it have a lot of applications. Its biggest application is for the wiring in aerospace and computer applications due to its excellent dielectric properties, e.g. as the insulator in cables and connector. In industry, PTFE can be applied in places where sliding action of parts is required due to its low friction such as plain bearings, gears and slide plates. PTFE is also applied in the production of carbon fiber composites and fiberglass composites. In addition, owing to its chemical inertia and high thermal resistance, PTFE is applied in places where acids, alkalis or other chemical are used, e.g. as the liner in industrial pipe lines.
Poly(tetrafluoroethylene) is prepared from tetrafluoroethylene and consists of repeating units in a predominantly linear chain:
F2C=CF2 --->[-CF2-CF2-]n
Tetrafluoroethylene polymer has the lowest coefficient of friction of any solid. It has remarkable chemical resistance and a very low brittleness temperature (100C). Its dielectric constant and loss factor are low and stable across a broad temperature and frequency range. Its impact strength is high.
http://pslc.ws/macrog/ptfe.htm
https://global.britannica.com/science/polytetrafluoroethylene
https://en.wikipedia.org/wiki/Polytetrafluoroethylene#Other
Tetrafl uoroethylene (TFE), also known as perfl uoroethylene, is a colorless, fl ammable, toxic gas. It is the monomer used for polytetrafl uoroethylene (PTFE), which is sold under the DuPont tradename of Tefl on. TFE is co-polymerized with other compounds to produce a variety of Tefl ons. TFE is produced by heating chlorodifl uoromethane (CHClF2, Freon-22) or trifl uoromethane (CHF3, Freon-23). TFE is used almost exclusively as a monomer in the production of PTFE. PTFE is a vinyl polymer, which means it is made from a monomer with carbon-carbon double bonds. PTFE is made from TFE by free radical polymerization.
PTFE’s unique physical properties are due to its chemical structure. PTFE consists of long
chains of carbon atoms surrounded by fluorine atoms. The fluorine atoms act as a protective
barrier that shields the carbon-carbon bond from chemical attack. The fluorine atoms repel
other atoms, making it diffi cult for anything to stick to PTFE. PTFE resins have very low
coeffi cients of friction (< 0.1). The strong fluorine-to-carbon bonds and high electronegativity
of fluorine make PTFE very stable. The long chains of PTFE pack closely together to give
a dense crystalline solid. The packing, which can be compared to stacking boards, produces
little cross-linking.
white powder, or white solid with a slippery feel
Polytetrafluoroethylene is a white solid with a waxy appearance and feel. It is
a tough flexible material of moderate tensile strength with a tendency to creep under compression.The electrical insulation properties are
outstanding and are nearly as good as those of polyethylene. The coefficient
of friction is unusually low and stated to be lower than that of any other solid;
the non-stick properties are also excellent.
Polytetrafluoroethylene has exceptional resistance to chemical attack and
is inert towards all types of reagents except molten alkali metals and fluorine.
No solvent is known for the polymer, but it is swollen by some fluorocarbon
oils at temperatures approaching the crystalline melting point; other organic
materials do not even swell the polymer.
Polytetrafluoroethylene has high thermal stability and retains its properties over a wide temperature range. The polymer may be used up to about
300°C for long periods without loss of strength and thin sections remain
flexible at temperatures below -100°C. There is some weight loss when
polytetrafluoroethylene is heated above about 200°C but this very small up
to about 350°C. The polymer also has good weather resistance.
The polymerized form of TFE was discovered accidentally by researchers at DuPont in 1938. During the mid-1930s, DuPont was investigating the development of new chlorofl uorocarbons (CFCs) for use as refrigerants. DuPont joined with General Motors to form a company called Kinetic Chemicals to combine their efforts in this area. Plunkett examined the properties of the substance. He discovered that the substance was inert to other chemicals, had a high melting point, and was very slippery. Plunkett also worked on duplicating the conditions necessary to produce it from TFE. He applied for a patent for polytetrafl uoroethylene polymers in 1939, which was granted in 1941 (U.S. Patent 2230654). He assigned the patent to Kinetic Chemicals. DuPont registered the term Tefl on in 1945 and introduced Tefl on products the next year.
Teflon is best known for its use in cookware, but its use in this area followed original industrial
applications in gaskets, sealers, tape, and electrical insulation. Th ese applications were a
direct result of the use of PTFE for military purposes during World War II.
polytetrafluoroethylene powder be used for plastic modification, average particle size is about 3-5 and 10-20 micrometer, PTFE has excellent performance, it has unique properties, including, no self-coagulation, no electrostatic effect, good intermiscibility, low molecular weight, good dispersion, high self-lubrication, low friction coefficient, etc.
PTFE (polytetrafluoroethylene) is a bulking agent, it is also used in cosmetic preparations to improve the formulation’s feel and spreadability. PTFe may also have some waterproofing potential.
Poly(tetrafluoroethylene) is used in hundreds of applications in addition to cookware. Poly(tetrafluoroethylene) is used in inks, plastics, coatings, and lubricants.Poly(tetrafluoroethylene) can be molded into gaskets, seals, bearings, gears, and other machine parts. Poly(tetrafluoroethylene) is used as liners, insulation, membranes, and adhesives. Tefl on tape is commonly used in plumbing work. Poly(tetrafluoroethylene) is used to produce rainproof garments.
For hookup and hookup-type wire in electronic equipment; in computer wire, electrical tape, electrical components, spaghetti tubing. Seals and piston rings, basic shapes, bearings, mechanical tapes, coated glass fabrics. As tubing and sheets for chemical laboratory and process work; for lining reaction vessels; for gaskets and pump packings, sometimes mixed with graphite or glass fibers; as electrical insulator especially in high frequency applications; filtration fabrics; protective clothing. Prosthetic aid.
A synthetic
polymer made from tetrafluoroethene (i.e.
CF2:CF2). It is able to withstand high temperatures
without decomposing and also
has a very low coefficient of friction, hence
its use in non-stick pans, bearings, etc.
The preferred commercial method of preparation of polytetrafluoroethylene
(PTFE) is by suspension polymerization. The details of the procedures
employed have not been disclosed but it appears that two main processes are
in use. In the first process, the conventional techniques of suspension poly�merization are used to produce a granular product suitable for moulding and
extrusion. The tetrafluoroethylene is polymerized under pressure in stainless
steel autoclaves with a free radical initiator such as ammonium persulphate.
The reaction is rapid and exothermic and requires careful control. The
polymer granules are collected, washed and dried. In the second process,
conditions are adjusted to give a dispersion of polymer of much finer particle
size and lower molecular weight. The product may be stabilized and employed in latex form in such uses as film casting, coating and impregnation of
fibres. Alternatively, the product may be coagulated to give a powder (often
called 'dispersion polymer') used mainly for the extrusion of thin flexible
sections.
Polytetrafluoroethylene is generally made from tetrafluoroethylene
gas by free-radical polymerization under pressure
with oxygen, peroxides, or peroxydisulfates. The “granular
resins” have medium-size particles that range from 30 to
600 mm. Colloidal aqueous dispersions, made by a different
process, are concentrated to about 60% by weight of the
polymer and have particles that average about 0.2 mm.
Coagulated dispersions with agglomerates that average
450mm are also available .
The use of PTFE as a release agent in coatings and certain
other food contact applications is permitted under FDA
regulations .
ChEBI: A polymer composed of repeating tetrafluoroethyl groups.
Poly(tetrafluoroethylene) is a fluoropolymer that is commercially known as PTFE. Its properties include high thermal stability, excellent chemical resistance, low dielectric constant and low surface energy. It is a hydrophobic polymer that is majorly used as a protective coating on the metal surface.
Evolves toxic fumes on heating. Question-
able carcinogen.
Fumes of heated polytetrafluo�roethylene (PTFE) cause polymer fume fever,
an influenza-like syndrome.
The polymer is insoluble, resistant to heat (upto 275°C) and chemical attack, and, in addition,has the lowest coefficient of friction of any solid.Because of its resistance to heat, the fabricationof polytetrafluoroethylene requires modificationof conventional methods. After molding thepowdered polymer using a cold press, the moldingsare sintered at 360 to 400°C by proceduressimilar to those used in powder metallurgy. Thesintered product can be machined or punched.Extrusion is possible if the powder is compoundedwith a lubricating material. Aqueoussuspensions of the polymer can also be used forcoating various articles. However, special surfacetreatments are required to ensure adhesionbecause polytetrafluoroethylene does not adherewell to anything.
Polytetrafluoroethylene (TFE resin) is usefulfor applications under extreme conditions ofheat and chemical activity. Polytetrafluoroethylenebearings, valve seats, packings, gaskets,coatings, and tubing can withstand relativelysevere conditions. Fillers such as carbon, inorganicfibers, and metal powders may be incorporatedto modify the mechanical and thermalproperties.
Because of its excellent electrical properties,polytetrafluoroethylene is useful when adielectric material is required for service at ahigh temperature. The nonadhesive quality isoften turned to advantage in the use of polytetrafluoroethyleneto coat articles such as rollsand cookware to which materials might otherwiseadhere.
The finished polymerized compound is inert under ordinary condtions. There have been reports of “polymer fume fever” in humansexposed to pyrolysis products, whch also are irritants. Smohng should be prohibited in areas where ths material is being fabricated or, in general, where there may be dust from it. Exposure to pyrolysis or decomposition products appears to be the chief health-related problem. Questionable carcinogen with experimental tumorigenic data by implant. Incompatible with fluorine, sodmm potassium alloy. Under the proper conditions it undergoes hazardous reactions with boron, magnesium, or titanium. When heated to above 750°F it decomposes to yield highly toxic fumes of F-.
Animal studies have not demonstrated
that this polymer is carcinogenic. No data are
available in humans. The polymer is not classifiable as to
its human carcinogenicity. However, the EPA found that
perfluorooctanoic acid (PFOA), a chemical used to produce
PTEE is a “likely carcinogen.
PTFE is the most stable of all TFE polymers and under physiological
conditions does not release any components (IARC
Monograph 74, 1999). PTFE is very inert chemically; only alkali
metals and fluorine under pressure attack PTFE (Hazardous
Substances Data Bank (HSDB)). There are no known ecotoxicological
effects for PTFE (DuPont MSDS, 2011).
There is no apparent mechanism of toxicity for orally administered
PTFE as no toxicologically significant effects were
observed following oral administration to rats for up to
90 days. The lack of toxicity is most likely due to the following:
(1) gastrointestinal absorption of PTFE is negligible given its
extremely high molecular weight (1 000 000–10 000 000 for
PTFE fine powder); (2) PTFE is chemically inert under physiologic
conditions; and (3) PTFE is not metabolized (Donovan
et al., 1990; Kim, 1996; Veber et al., 2002). The mechanism
of action of subcutaneously injected PTFE in mice is attributed
to localized inflammation consistent with a foreignbody
response; similar effects were seen following subareolar
injection in rabbits and dogs, and periurethral injections
in dogs.
When PTFE is heated or exposed to temperatures ≥200°C,
it will decompose and release toxic vapors that cause polymerfume
fever in humans.