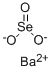Barium selenite has the molecular formula of BaSeO3
and the molecular weight of 264.2865 g/mol. Its CAS
number is 13718-59-7. It is a white solid that is insoluble
in water. It can be prepared by the addition of sodium
selenite to a solution of barium chloride:
BaCl2(aq)+ Na2SeO3(aq) ? BaSeO3(solid)
It can also be prepared by treating a solution of
barium chloride with selenous acid and adding sodium
carbonate to start precipitation. There is no record of
a hydrate and the anhydrate is stable upon heating to
over 950 °C.
The heat of formation of barium selenite is:
△fH298 - BaSeO3 = 2230.6 kcal/mol (—964.7 kJ/mol).
The precipitate of barium selenite is not oxidized by
air during drying at 110°C. In air, however, it oxidizes
to the selenate at about 700–750°C:
2BaSeO3+ O2+ heat ? 2BaSeO4
Bariumselenite is a fine white crystalline powder that
is mainly used in the glass industry to color and decolor
glass.
Barium selenite is primarily chosen for producing
special glass and leads to the formation of glass with
high index of refraction. Barium selenite has weak
oxidizing or reducing powers. Redox reactions can
however still occur. The majority of compounds in this
class are slightly soluble or insoluble in water. Noncombustible,
the substance itself does not burn but may
decompose upon heating to produce corrosive and/or
toxic fumes of SeO2.
Barium selenite has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive. Noncombustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes.
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Flammability and Explosibility
Not classified