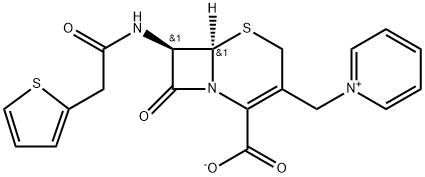
This was derived by adding two side chains to the nucleus of
cephalothin, and has the formula 7-(2-thienyl acetamido)-3-(1-pyridylmethyl)-3-cephem-4-carboxylic acid betaine (Muggleton et al.,
1964). Cephaloridine was initially widely used, but it had two main
disadvantages. It was an unreliable antistaphylococcal drug, as it was
relatively easily hydrolyzed by Staphylococcus aureus beta-lactamase
(Laverdiere et al., 1978; Sabath, 1989). Second, cephaloridine was
nephrotoxic (Foord, 1975; Appel and Neu, 1977). Marketing of the
drug was discontinued in the USA in December 1980. Nowadays, this
drug is used very rarely, if at all.
ChEBI: A cephalosporin compound having pyridinium-1-ylmethyl and 2-thienylacetamido side-groups. A first-generation semisynthetic derivative of cephalosporin C.
7-Aminocephalosporanic acid (5.00 g) which passed through a 100-mesh
sieve was suspended in boiling ethyl acetate (200 ml), and 2-thienylacetyl
chloride (Cagniant, Bull. Soc. Chim. France, 1949, 847) (4.42 g, 1.5 equiv.)
was added in ethyl acetate (20 ml). The mixture was boiled under reflux for
40 minutes, cooled, and filtered. Aniline (5.03 ml) was added, and after 1
hour the mixture was extracted with 3% sodium hydrogen carbonate solution
(1 x 150 ml, 2 x 100 ml, 1 x 50 ml) and the alkaline extracts washed with
ethyl acetate (3 x 100 ml). The aqueous solution was acidified to pH 1.2, and
extracted with ethyl acetate (2 x 150 ml). The ethyl acetate extract was
washed with water (4 x 40 ml), dried (MgSO4), and concentrated in vacuo to
low volume. The crude 7-2'-thienylacetamidocephalosporanic acid (2.5 g)
which separated was collected by filtration. Evaporation of the filtrate gave a
further 2.68 g (71%) of the product, which was purified by crystallization from
ethyl acetate, then aqueous acetone, MP 150°C to 157°C (decomp.).
7-2'-Thienylacetamidocephalosporanic acid (7.0 g) was suspended in water
(60 ml) and stirred with pyridine (7 ml) until the acid dissolved. The resulting
solution (pH 5.9) was kept at 35°C for 3 days, then filtered and extracted with
methylene chloride (4 x 60 ml). The methylene chloride extract was back-extracted with a little water and the total aqueous solutions were then
percolated through a column of Dowex 1 x 8 resin, (100 to 200 mesh, 150 g)
in the acetate form at pH 4.3. The column was washed with water until the
optical rotation of the eluate fell to zero and the eluate (500 ml) was freeze-dried. The residual white solid was dissolved in the minimum volume of
methanol and after a few minutes the pyridine derivative crystallized; this is
the cephaloridine product.
Kefloridin (Lilly); Loridine
(Lilly);Cepalorin;Faredina;Latorex;Lauridin.
World Health Organization (WHO)
Cefaloridine, a semi-synthetic cephalosporin antibiotic, was
introduced into medicine in 1964 for the treatment of bacterial infections. It is
considered to be the most toxic of the cephalosporins, and for this reason is now
seldom used. Nevertheless, it still remains available in certain countries and the
World Health Organization is not aware of restrictive actions taken elsewhere.
Cephaloridine, along with cephalothin, was the first member of the synthetic cephalosporin C class of antibiotic, to be introduced clinically. It was synthesized, starting with 7-aminocephalosporanic acid, by Glaxo Research Laboratories in 1962. This drug shows strong activity against gram-positive and gramnegative bacteria and Leptospira, including benzylpenicillin-resistant strains. It has been given widely by intravenous, intramuscular, and intraspinal injection to treat a variety of infections caused by Staphylococcus, Streptococcus, Neisseria, Klebsiella, Escherichia coli, and other pathogens. Because of its renal toxicity and the development of newer and more active synthetic cephalosporins, its use is declining.
Moderately to very toxic.