Ethyl lactate, an ester of lactic acid, is a "green" solvent derived from processing corn. Lactate esters solvents are commonly used solvents in the paints and coatings industry and have numerous attractive advantages including being 100% biodegradable, easy to recycle, noncorrosive, noncarcinogenic, and nonozone depleting.
Ethyl lactate is an important organic ester, which is biodegradable and can be used as food additive, in perfumery, as flavour chemicals and solvent, which can dissolve acetic acid cellulose and many resins (Tanaka et al., 2002). It is a particularly attractive solvent for the coatings industry as a result of its high solvency power, high boiling point, low vapour pressure and low surface tension. Ethyl lactate is a desirable coating for wood, polystyrene and metals and also acts as a very effective paint stripper and graffiti remover. It has replaced solvents including N-methyl Pyrrolidone (NMP) (Reisch, 2008), toluene, acetone and xylene, which has resulted in the workplace being made a great deal safer.
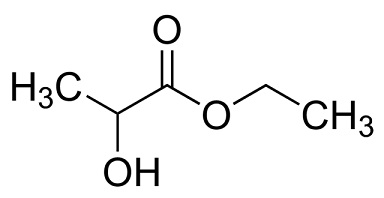
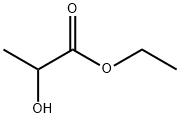
ethyl lactate structure
Ethyl lactate appears as colorless to light yellow transparent liquid with rum, fruit and cream aroma. The freezing point:-25°C; the boiling point: 154°C, specific rotation [a] 14d:-10°. It is easily soluble in ethanol, acetone, ether, esters and other organic solvents; there is some degree of hydrolysis upon being miscible with water. Mouse oral LD50: 2.5g/kg, ADI is subject to no special provisions (FAO/WHO, 1994).

Ethyl lactate is a common solvent in pharmaceutical, cosmetic, detergent, coatings, food and fragrance production. It is considered biodegradable in its natural form and it is exempt from many restrictions imposed in accordance with the disposal of chemicals. For this reason it is a highly desirable product in a “green”, environmentally conscious industry. In the presence of water, acids and bases the chemical will hydrolyse into ethanol and lactic acid.
Ethyl Lactate is a solvent manufactured from l (+) lactic acid which is miscible in water and most organic solvents and is cleared for use as a flavoring agent. it is a naturally occurring constituent of california and spanish sherries. it is a clear, colorless, nontoxic liquid of low volatility, having a ph of 7–7.5. it is used as a food and beverage flavoring agent.Ethyl lactate is used in the electronics industry to remove salts and fat from circuit boards; it is also a component in paint strippers.
Ethyl lactate is usually produced by the esterification of lactic acid with ethanol using sulfuric acid catalyst.
FEMA (mg/mL): Soften drink: 5.4; Cold drinks: 17; Candies 28; baked goods 71; pudding class 8.3; gums 580 to 3100; alcohol 1000; syrup 35.
Take moderate as limit (FDA § 172.515, 2000).
ADI is not subject to specific special provisions (FAO/WHO, 1994).
GRAS (FEMA).
LD50 2500 mg/kg (mouse, oral).
Ethyl Lactate is a colorless liquid with a light ethereal, buttery odor. It miscible in water, alcohols, ketones , esters, hydrocarbons, ether and oil. The odor threshold is reported to be 0.89 mg/m3 and the odor nuisance threshold to be 65 mg/m3.
Ethyl lactate is reported to be found in apple, citrus fruits, pineapple, peas, sauerkraut, vinegar, bread, beer, grape brandy, rum, whisky, cider, sherry, wine, cocoa, banty beer, plum brandy and pear brandy. It has been approved by the US FDA for food use.
Ethyl lactate is used as a solvent for nitrocellulose, cellulose acetate, and many cellulose ethers and resins. It is also used in lacquers, paints, enamels, varnishes, stencil sheets, safety glass and flavoring. Ethyl lactate is furthermore used in some cosmetic formulations in soaps, detergents, creams and lotions with a maximum concentration of 0.2 %. In recent years it has been used as degreaser as a substitute for trichloroethylene.
ChEBI: Ethyl 2-hydroxypropanoate is the ethyl ester obtained of 2-hydroxypropanoic acid. It has a role as a metabolite. It is a secondary alcohol, a lactate ester and an ethyl ester. It is functionally related to a 2-hydroxypropanoic acid.
Ethyl lactate is produced by the esterification of lactic acid with ethanol in the presence of a little mineral oil, or by combination of acetaldehyde with hydrocyanic acid to form acetaldehyde cyanhydrin. This is followed by treatment with ethanol (95%) and hydrochloric or sulfuric acid. Purification is achieved using fractional distillation. The commercial product is a racemic mixture.
A clear colorless liquid with a mild odor. Flash point 115°F. Denser than water and soluble in water. Vapors heavier than air.
Flammable. Soluble in water.
Ethyl lactate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Inhalation of concentrated vapor may cause drowsiness. Contact with liquid causes mild irritation of eyes and (on prolonged contact) skin. Ingestion may cause narcosis.
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Ethyl lactate is used as a flavoring agent in pharmaceutical
preparations, and is found in food products. The estimated
acceptable daily intake for lactic acid is 12.5 mg/kg body-weight.
In general, lactate esters have an oral LD50 > 2000 mg/kg; and
the inhalation LC50 is generally above 5000 mg/m3. They have the
potential of causing eye and skin irritation (on prolonged contact),
but not sensitization. Ethyl lactate is moderately toxic by
intraperitoneal, subcutaneous, and intravenous routes. There is
low oral and skin contact toxicity; although ingestion may cause
nausea, stomach and throat pain, and narcosis. Inhalation of
concentrated vapor of ethyl lactate may cause irritation of the
mucous membranes, drowsiness, and narcosis.
LD50 (rat, oral): >5.0 g/kg
LD50 (mouse, oral): 2.5 g/kg
LD50 (mouse, SC): 2.5 g/kg
LD50 (mouse, IV): 0.6 g/kg
LD50 (rabbit, skin): >5.0 g/kg
Veterinary Drugs and Treatments
Ethyl lactate shampoo can be used when an antibacterial shampoo (bacteriostatic and bactericidal) is needed particularly in animals with
surface and superficial pyodermas that cannot tolerate benzoyl peroxide. It also has keratoplastic effect, which provides anti-seborrheic
activity.
A lipid soluble compound, ethyl lactate penetrates hair follicles and sebaceous glands where bacterial lipases convert it into lactic acid
and ethanol, which are responsible for its antibacterial action. It is not as active as benzoyl peroxide against staphylococcal organisms, but
is less irritating and drying.
Stable at normal temperature and pressure. Ethyl lactate is a
flammable liquid and vapor. Store in a cool, dry, and well-ventilated
location away from any fire hazard area, in a tightly closed
container.
Incompatible with bases or strong alkalis and may cause fire or
explosion with strong oxidizing agents.
GRAS listed. Reported in the EPA TSCA Inventory.