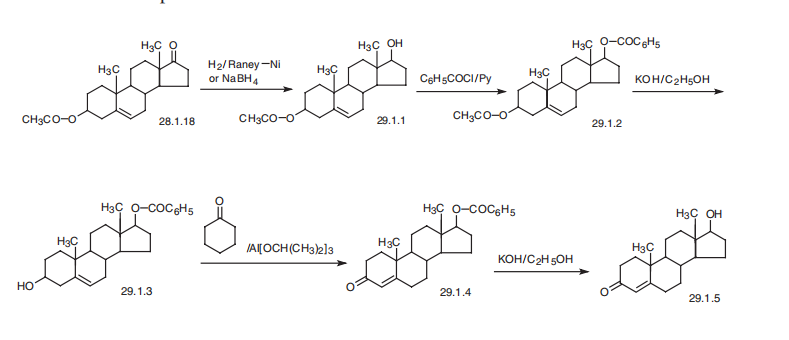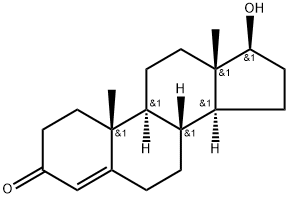
Testosterone
- Product NameTestosterone
- CAS58-22-0
- MFC19H28O2
- MW288.43
- EINECS200-370-5
- MOL File58-22-0.mol
Chemical Properties
| Melting point | 152-156 °C |
| alpha | 101 º (c=1, dioxane 25 ºC) |
| Boiling point | 370.65°C (rough estimate) |
| Density | 1.0484 (rough estimate) |
| refractive index | 1.4709 (estimate) |
| Flash point | 5 °C |
| storage temp. | 2-8°C |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: soluble18.2 mg/ml |
| pka | 15.06±0.60(Predicted) |
| form | powder |
| Water Solubility | 22.79mg/L(20 ºC) |
| Merck | 13,9255 |
| BRN | 1915399 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChIKey | MUMGGOZAMZWBJJ-DYKIIFRCSA-N |
| CAS DataBase Reference | 58-22-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Testosterone(58-22-0) |
| EPA Substance Registry System | Testosterone (58-22-0) |
Safety Information
| Hazard Codes | T,Xn,F |
| Risk Statements | 60-61-11-19-20-40-63-45-36-20/21/22-38 |
| Safety Statements | 53-45-24/25-36/37-26-16 |
| RIDADR | UN 2252 3/PG 2 |
| WGK Germany | 3 |
| RTECS | XA3030000 |
| HS Code | 29372900 |
| Hazardous Substances Data | 58-22-0(Hazardous Substances Data) |
| Toxicity | LD50 oral in mammal (species unspecified): > 5gm/kg |