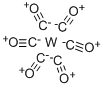
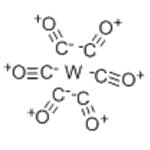
Tungsten hexacarbonyl is easily vaporized and decomposed by the electron beam — providing a convenient source of tungsten atoms. This product is widely used in electron beam-induced deposition processes, it is also used as a precursor to catalysts for alkene metathesis and to desulfurize organosulfur compounds. It is relatively air-stable, and it is sparingly soluble in non-polar organic solvents. W(CO)6 reactions begin with the displacement of some of its CO ligands. Similar to Mo(CO)6 in behavior, W(CO)6 typically forms compounds that are kinetically more robust. Synthesis of this product are reported (see the links below).
Tungsten hexacarbonyl, [W(CO)6] may be used as a precursor for the deposition of WO3-x films, which may be used as gas sensors for the detection of NO2.
Precursor to a Fischer carbene complex which reduces Pd(II) to nanoparticulate Pd(0) that is catalytically active in Hiyama cross-coupling.
Volatile starting material used for the atomic layer deposition of tungsten oxide, tungsten nitride.
Tungsten hexacarbonyl is used to produce tungsten coatings on base metals. This is done by deposition of the carbonyl on the metal surface, which decomposes to leave a tungsten coating.
Tungsten hexacarbonyl is produced by heating tungsten metal with carbon monoxide at high pressure. Also, carbonyl can be prepared by reducing the tungsten hexachloride by heating with iron powder under carbon monoxide pressure.
White, volatile, highly refractive, crystalline
solid. Decomposes without melting at 150C.
One of the more stable carbonyls. Insoluble in water; soluble in
organic solvents.
White crystalline solid; density 2.65 g/cm3; decomposes at 170°C without melting; sublimes; vapor pressure 0.1 torr at 20°C; insoluble in water; soluble in most organic solvents.
It is used as a catalyst in many organicsynthetic reactions.
Tungsten coatings on base metals by deposition
and decomposition of the carbonyl.
Tungsten Hexacarbonyl is an useful catalyst in carbonyation amines, as well as an stable source of tungsten atoms through electron beam-induced depostion.
Atomic number of base material: 74 Tungsten
Toxicity of this compound is not reported.Although air stable at ambient temperature,upon heating it emits toxic carbonmonoxide. Chronic exposure to its vaporsor dusts can cause bronchitis. Ingestion islikely to produce the toxic effect of tungstenoxides.
It explodes when heated to high temperature.
Solution in ether can explode if stored for a
long time.
Sublime it in vacuo before use [Connoe et al. J Chem Soc, Dalton Trans 511 1986]. TOXIC.