2,4,6-Collidine is an reagent used for various synthetic preparations such as the synthesis of methylated pyridines by three-componet catalytic condensation of acetylene, acetone and ammonia.
2,4,6-Collidine is used as a tissue fixative for electron microscopy. It is useful in dehydrohalogenation reactions and acts as a solvent for the cleavage of hindered esters by anhydrous lithium iodide.
Methyl, ethyl, propyl, and trimethyl homologs of
pyridine.
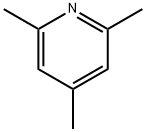
2,4,6-Trimethylpyridine is a pyridine derivative. It has a pK of 7.4. The product can react with trifluoroiodomethane in cyclopentane solution to afford 1:1 complex. This complex was investigated by NMR (Nuclear Magnetic Resonance) spectroscopy. Collidine-buffered osmium tetroxide solutions have been prepared by adding osmium tetroxide solution to it. These solutions have been used as fixative for electron microscopic studies.
2,4,6-Trimethylpyridine can undergo oxidation with potassium permanganate to form 2,4,6-pyridinetricarboxylic acid.
Commercial samples may be grossly impure. Likely contaminants include 3,5-dimethylpyridine, 2,3,6-trimethylpyridine and water. Brown, Johnson and Podall [J Am Chem Soc 76 5556 1954] fractionally distilled 2,4,6-trimethylpyridine under reduced pressure through a 40cm Vigreux column (p 11) and added to 430mL of the distillate slowly, with cooling to 0o, 45g of BF3-diethyl etherate. The mixture was again distilled, and an equal volume of dry *benzene was added to the distillate. Dry HCl was passed into the solution, which was kept cold in an ice-bath, and the hydrochloride was filtered off. It was recrystallised from absolute EtOH (1.5mL/g) to m 286-287o[m 256o(sealed tube), also m 293-294o subliming slowly]. The free base was regenerated by treatment with aqueous NaOH, then extracted with *benzene, dried (MgSO4) and distilled under reduced pressure. Sisler et al. [J Am Chem Soc 75 446 1953] precipitated trimethylpyridine as its phosphate from a solution of the base in MeOH by adding 85% H3PO4, shaking and cooling. The free base was regenerated as above. Garrett and Smythe [J Chem Soc 763 1903] purified the trimethylpyridine via the HgCl2 complex. It is more soluble in cold than hot H2O [the solubility is 20.8% at 6o, 3.5% at 20o, 1.8% at 100o]. Alternatively, purify it by dissolving it in CHCl3, adding solid K2CO3 and Drierite, filtering and fractionally distilling through an 8in helix-packed column. The sulfate has m 205o, and the picrate (from hot H2O) has m 155-156o. [Frank & Meikle J Am Chem Soc 72 4184 1950, Beilstein 20 H 250, 20 I 87, 20 II 164, 20 III/IV 2810, 20/6 V 93.]