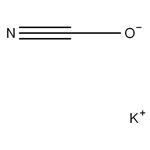
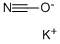Potassium cyanate, KCNO, white solid, soluble, formed along with lead metal by reaction of potassium cyanide and lead monoxide solids upon heating. Source of cyanate.
Potassium cyanate, KOCN,is colorless,water soluble crystals. Used as an herbicide and for the manufacture of drugs and organic chemicals,
Herbicide, manufacture of organic chemicals
and drugs, treatment of sickle-cell anemia.
Potassium cyanate is used as an inhibitor to prevent sickling of erythrocytes and has potential use as a treatment for sickle-cell anemia. Potassium cyanate is also used as a reagent to carbamylate proteins.
Potassium cyanate is used as chemical intermediate and
as a weed killer. It is also used for the heat treatment of
metals.
ChEBI: Potassium cyanate is a cyanate salt and a one-carbon compound. It has a role as a herbicide.
Potassium cyanate can be prepared by reacting urea with alkali hydroxide or carbonates at high temperatures.
Flammability and Explosibility
Not classified
Poison by
intraperitoneal route. Moderately toxic by
ingestion. Causes irritation of the
gastrointestinal tract. An herbicide. It is said
to be slowly metabolized in the body to
cyanide but does not have high toxicity of
cyanides. When heated to decomposition it
emits very toxic fumes of CNand K2O.
Common impurities include ammonia and bicarbonate ion (from hydrolysis). Purify it by preparing a saturated aqueous solution at 50o, neutralising with acetic acid, filtering, adding two volumes of EtOH and keeping for 3-4hours in an ice bath. (More EtOH can lead to co-precipitation of KHCO3.) Filter, wash it with EtOH and dry it rapidly in a vacuum desiccator (P2O5). The process is repeated [Vanderzee & Meyers J Chem Soc 65 153 1961].