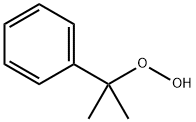
Cumene hydroperoxide, an organic peroxide,is a colorless to pale yellow to green liquid. Mild odor.Molecular weight=152.21; Boiling point=153℃;Freezing/Melting point=2 10℃. It explodes on heating;Flash point=79℃. Its explosive limits are: LEL=0.9%;UEL=6.5%. Hazard Identification (based on NFPA-704 MRating System): Health 1, Flammability 2, Reactivity 4(Oxidizer). Slightly soluble in water.
Cumene hydroperoxide, an organic peroxide, is a colorless to pale yellow to green liquid. Mild odor.
Cumene hydroperoxide is used in the preparation of polystyrene nanocapsules. It acts as a curing agent for polyester resins and as an oxidizer in organic chemical reactions. It serves as an initiator for radical polymerization especially for acrylate and methacrylate monomers. It also employed as an intermediate in the cumene process for developing phenol and acetone from benzene and propene. Further, it is used as an epoxidation reagent for allylic alcohols and fatty acid esters. In addition to this, it is also used to prepare methylstyrene, acetophenone and cumyl alcohol.
Cumene hydroperoxide is used for the manufactureof acetone and phenols; for studyingthe mechanism of NADPH-dependent lipidperoxidation; and in organic syntheses.
Production of acetone and phenol; polymerization catalyst, particularly in redox systems, used for
rapid polymerization.
ChEBI: A peroxol that is cumene in which the alpha-hydrogen is replaced by a hydroperoxy group.
Colorless to light yellow liquid with a sharp, irritating odor. Flash point 175°F. Boils at 153°C and at 100°C at the reduced pressure of 8 mm Hg. Slightly soluble in water and denser than water. Hence sinks in water. Readily soluble in alcohol, acetone, esters, hydrocarbons, chlorinated hydrocarbons. Toxic by inhalation and skin absorption. Used in production of acetone and phenol, as a polymerization catalyst, in redox systems.
Slightly soluble in water and oxidized in air at approximately 130°C.
Cumyl hydroperoxide is a strong oxidizing agent. May react explosively upon contact with reducing reagents Violent reaction occurs upon contact with copper, copper alloys, lead alloys, and mineral acids. Contact with charcoal powder gives a strong exothermic reaction. Decomposes explosively with sodium iodide [Chem. Eng. News, 1990, 68(6), 2]. Can be exploded by shock or heat [Sax, 2 ed., 1965, p. 643]. May ignite organic materials.
Toxic by inhalation and skin absorption.
Strong oxidizing agent; may ignite organic materials.
Cumene hydroperoxide is a mild to moderateskin irritant on rabbits. Subcutaneousapplication exhibited a strong delayed reactionwith symptoms of erythema and edema(Floyd and Stockinger 1958). Strong solutionscan irritate the eyes severely, affectingthe cornea and iris.
Its toxicity is comparable to that of tertbutylhydroperoxide. The toxic routes areingestion and inhalation. The acute toxicitysymptoms in rats and mice were muscleweakness, shivering, and prostration.Oral administration of 400 mg/kg resulted inexcessive urinary bleeding in rats.
LD50 value, oral (rats): 382 mg/kg
LD50 value, intraperitoneal (rats): 95 mg/kg
Although cumene hydroperoxide is toxic,its pretreatment may be effective against thetoxicity of hydrogen peroxide. In humans, itstoxicity is low.
Cumene hydroperoxide is mutagenic andtumorigenic (NIOSH 1986). It may causetumors at the site of application. In mice,skin and blood tumors have been observed.Its cancer-causing effects on humans are notknown.
Inhalation of vapor causes headache and burning throat. Liquid causes severe irritation of eyes; on skin, causes burning, throbbing sensation, irritation, and blisters. Ingestion causes irritation of mouth and stomach.
Flammable; highly reactive and oxidizing.
Flash point 79°C (174.2°F); vapor density
5.2 (air= 1); autoignition temperature not
reported; self-accelerating decomposition
temperature 93°C (199.4°F).
When exposed to heat or flame, it may
ignite and/or explode. A 91–95% concentration
of cumene hydroperoxide decomposes
violently at 150°C (302°F) (NFPA 1986).
Duswalt and Hood (1990) reported violent
decomposition when this compound mixed
accidentally with a 2-propanol solution of
sodium iodide.
It forms an explosive mixture with air.
The explosive concentration range is not
reported. Hazardous when mixed with easily
oxidizable compounds. Fire-extinguishing
agent: water from a sprinkler or fog nozzle
from an explosion-resistant location.
Flammability and Explosibility
Non flammable
Cumene hydroperoxide is used as polymerization initiator, curing agent for unsaturated polyester resins and cross-linking agent; as an intermediate in the process for making phenol plus acetone from cumene.
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, beginrescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR if heartaction has stopped. Transfer promptly to a medical facility.When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Donot make an unconscious person vomit. Medical observationis recommended for 24-48 h after breathing overexposure,as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
Cumene hydroperoxide is stored in a cool,dry and well-ventilated area isolated fromother chemicals. It should be protectedagainst physical damage. It may be shippedin wooden boxes with inside glass or earthenwarecontainers or in 55-gallon metal drums.
UN3109 Organic peroxide type F, liquid, Hazard Class: 5.2; Labels: 5.2-Organic peroxide, Technical Name Required.
Purify the hydroperoxide by adding 100mL of 70% material slowly and with agitation to 300mL of 25% NaOH in water, keeping the temperature below 30o. The resulting crystals of the sodium salt are filtered off, washed twice with 25 mL portions of *benzene, then stirred with 100mL of *benzene for 20minutes. After filtering off the crystals and repeating the washing, they are suspended in 100mL of distilled water and the pH is adjusted to 7.5 by addition of 4M HCl. The free hydroperoxide is extracted into two 20mL portions of n-hexane, and the solvent is evaporated under vacuum at room temperature, the last traces being removed at 40-50o/1mm [Fordham & Williams Canad J Res 27B 943 1949]. Petroleum ether, but not diethyl ether, can be used instead of *benzene, and powdered solid CO2 can replace the 4M HCl. [Beilstein 6 IV 3221.] The material is potentially EXPLOSIVE.
The pure material is reported to explode on heating at elevated temperatures (various values given are 50°, 109, 150°C) or in strong sunlight. The substance is a strong oxidizer; reacts violently with combustible and reducing agents, causing fire and explosion hazard. Contact with metallic salts of cobalt, copper or lead alloys; mineral acids; bases; and amines may lead to violent decomposition. Vapor forms an explosive mixture with air. May accumulate static electrical charges, and may cause ignition of its vapors.