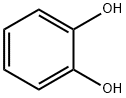
Catechol is a white crystalline solid. Turnsbrown on contact with light and air. Molecularweight=110.12; Specific gravity (H2O:1)=1.3; Boilingpoint=245.5℃; Freezing/Melting point=104℃. It sublimes readily; Vapor pressure=10 mmHg at 117℃; Flashpoint=127℃; Autoignition temperature=512℃.Explosive Limits in air: LEL=1.4%; UEL-Unknown.Hazard Identification (based on NFPA 704 M RatingSystem): Health 3, Flammability 1, Reactivity 2. Solubilityin water=44%.
Catechol also called Pyrocatechol is a white crystalline solid. Turns
brown on contact with light and air.
Catechol is used in photography, in dyeing fur, and as a topical antiseptic.
In the manufacture of rubber antioxidants
and monomer inhibitors to stop radical
polymerization; in dyes, as a photographic
developer; in formulations for pharmaceuticals,
perfumes, inks, and insecticides
In photography; dyeing fur; as reagent.
A colourless crystalline
PHENOL containing two hydroxyl groups. It
is used in photographic developing.
Catechol may be obtained by the fusion of o-phenolsulfonic
acid with alkali, by heating chorophenol with a
solution of sodium hydroxide at 200°C in an autoclave, or
by cleavage of the methyl ether group of guaiacol (obtained
from beechwood tar) with hydriodic acid.
Solid; white; odorless. Sinks and mixes with water.
Turns brown on exposure to air and light, especially when moist. Water soluble. Aqueous solutions soon turn brown on exposure to air and light.
POISONOUS GASES MAY BE PRODUCED WHEN HEATED. Pyrocatechol may form toxic fumes at high temperatures. [USCG, 1999]. Pyrocatechol can react with acid chlorides, acid anhydrides, bases and oxidizing agents. Pyrocatechol reacts violently on contact with concentrated nitric acid. Pyrocatechol acts as a reducing agent .
Strong irritant. Toxic by skin absorption.
Eye and upper respiratory tract irritant, and der-
matitis. Possible carcinogen.
DUST: Irritating to eyes, nose and throat. If inhaled will cause coughing or difficult breathing. SOLID: Will burn skin and eyes. Harmful if swallowed.
Acute oral and percutaneous toxicity of pyrocatechol is greater than that of phenol; inhalation toxicity is less than that of phenol. The toxic symptoms include weakness, muscular pain, dark urine, tremor, dyspnea, and convulsions. Large amounts can produce degenerative changes in renal tubules. Large doses can cause death due to respiratory failure. Skin contact can cause eczematous dermatitis.
LD50 value, oral (rats): 260 mg/kg
LD50 value, skin (rabbits): 800 mg/kg.
Combustible. POISONOUS GASES MAY BE PRODUCED WHEN HEATED. May form toxic fumes at high temperatures.
Flammability and Explosibility
Non flammable
Poison by ingestion,
subcutaneous, intraperitoneal, intravenous,
and parenteral routes. Moderately toxic by
skin contact. Experimental reproductive
effects. Can cause dermatitis on skin
contact. An allergen. Human mutation data
reported. Questionable carcinogen. Systemic
effects sirmlar to those of phenol.
Combustible when exposed to heat or
flame; can react vigorously with oxidizing
materials. Hypergolic reaction with
concentrated nitric acid. To fight fire, use
water, CO2, dry chemical. When heated to
decomposition it emits acrid smoke and
irritating fumes. See also PHENOL.
Used as a chemical intermediate;
pharmaceutical and veterinary drug; as an antiseptic; in
photography; in dyestuff manufacture and application. It is
also used in electroplating, in the formulation of specialty
inks; in antioxidants; and light stabilizers.
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Note to physician: Treat for methemoglobinemia.Spectrophotometry may be required for precise determination of levels of methemoglobinemia in urine.
Catechol has been extensively
studied for its role in carcinogenesis of the rat glandular
stomach; it was concluded that pyrocatechol is
carcinogenic. When rats and mice were administered
0.8% pyrocatechol in their feed for life, there was an increase
in glandular stomach adenocarcinoma in both male and
female rats. Pyrocatechol also caused hyperplasia of the
glandular stomach in both rats and mice, a mechanism
that could cause promotion of carcinogen-initiated
cells; no effects on the esophagus or urinary bladder
were reported. There were no cutaneous neoplasms when
pyrocatechol was applied in dermal studies. Catechol
may be classified as a cocarcinogen because it enhanced the
number and/or incidence of lesions in the stomach induced
by several carcinogenic nitrosamines and cutaneous neoplasms
when administered dermally together with several
carcinogens.
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with catecholyou should be trained on its proper handling and storage.Before entering confined space where this chemical may bepresent, check to make sure that an explosive concentrationdoes not exist. Store in tightly closed containers in a cool,well-ventilated area away from strong oxidizers and acids.Use only nonsparking tools and equipment, especially whenopening and closing containers of this chemical. Sources ofignition, such as smoking and open flames, are prohibitedwhere this chemical is used, handled, or stored in a mannerthat could create a potential fire or explosion hazard.
UN 2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required.
Crystallise catechol from *benzene or toluene and sublime it in vacuo. [Rozo et al. Anal Chem 58 2988 1986, Beilstein 6 IV 5557.]
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides.