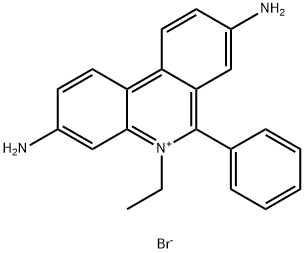
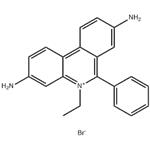
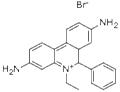
Ethidium Bromide (EtBr), commonly used in research laboratories as a stain for the visualization of nucleic acids in electrophoresis gels, is a toxic chemical and a potent mutagen. When used in nucleic acid staining, ethidium bromide fluoresces a red-orange to pink color under ultraviolet light and with increased fluorescence when bound to double-stranded DNA. While it is not specifically regulated as a hazardous waste, the mutagenic properties may present health hazards and disposal concerns if it is not managed properly in the laboratory.
Ethidium bromide (EtBr) is a fluorescent dye widely used in molecular biology research. Early usage was as a veterinary trypanocide. It is a mutagenic compound that intercalates double-stranded DNA and RNA. The fluorescence of EtBr increases 21-fold upon binding to double-stranded RNA, 25-fold on binding double-stranded DNA (although histones block binding of EtBr to DNA). Ethidium bromide has been used in multiple fluorimetric assays for nucleic acids. It has been shown to bind to single-stranded DNA (although not as strongly) and triple-stranded DNA. Because of the binding to DNA, EtBr is a powerful inhibitor of DNA polymerase.
Molecular Biology-grade Powder is suitable for use in gel electrophoresis and DNA isolation procedures.
Aqueous Solution (10 mg/mL) is suitable for use in gel electrophoresis and DNA isolation procedures.
Molecular Biology-grade Aqueous Solution (500 mg/mL) is suitable for use in gel electrophoresis.
EtBr is a potent mutagen (may cause genetic damage), and moderately toxic after an acute exposure. EtBr can be absorbed through skin, so it is important to avoid any direct contact with the chemical. EtBr is an irritant to the skin, eyes, mouth, and upper respiratory tract. It should be stored away from strong oxidizing agents in a cool, dry place, and the container must be kept undamaged and tightly closed.
Purple/maroon crystalline powder
Ethidium bromide (EtBr) is the most commonly used nucleic acid stain for PAGE or agarose gel electrophoresis. The fluorescence of EtBr increases 21-fold upon binding to double-stranded RNA and 25-fold on binding double-stranded DNA so that destaining the background is not necessary with a low stain concentration (10 μg/ml).
Ethidium bromide has been used in a number of fluorimetric assays for nucleic acids. It has been shown to bind to single-stranded DNA (although not as strongly) and triple-stranded DNA. Because of its ability to bind to DNA, EtBr is an inhibitor of DNA polymerase. Antiprotozoal (Trypanosoma).
antiprotozoal, intercalcates with DNA
Intercalating agent and fluorescent label for DNA.
ChEBI: Ethidium bromide is the organic bromide salt of ethidium. It has a role as a trypanocidal drug, a geroprotector and an intercalator. It contains an ethidium.
Acute toxic effects from exposure to ethidium bromide have not been thoroughly
investigated. Ethidium bromide is irritating to the eyes, skin, mucous membranes,
and upper respiratory tract.
Although there is no evidence for the carcinogenicity or teratogenicity of this
substance in humans, ethidium bromide is strongly mutagenic and therefore should
be regarded as a possible carcinogen and reproductive toxin
Flammability and Explosibility
Ethidium bromide does not pose a flammability hazard (NFPA rating = 1).
Ethidium bromide intercalates double-stranded DNA and RNA and acts as a frameshift mutagen. It can also be used in conjunction with acridine orange to differentiate between viable, apoptotic and necrotic cells.
Because ethidium bromide can bind with DNA, it is highly toxic as a mutagen. It may potentially cause carcinogenic or teratogenic effects, although no scientific evidence showing either health effect has been found. Exposure routes of ethidium bromide are inhalation, ingestion, and skin absorption. An acute exposure to ethidium bromide causes irritation of the mouth, upper respiratory tract, skin, and eyes.
Because of its mutagenicity, stock solutions of this compound should be prepared in a fume hood, and protective gloves should be worn at all times while handling this substance. Operations capable of generating ethidium bromide dust or aerosols of ethidium bromide solutions should be conducted in a fume hood to prevent exposure by inhalation.
Crystallise it from MeOH or EtOH [Lamos et al. J Am Chem Soc 108 4278 1986]. Its solubility in H2O is 1%. [Beilstein 22/11 V 352.] POSSIBLE CARCINOGEN.
No incompatibilities are known.
Excess ethidium bromide and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.