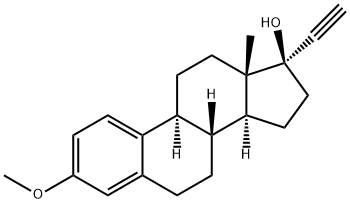
Pharmacological estrogen molecule
Mestranol is an orally active estrogenic steroid. It was the estrogen used in many of the first oral contraceptives
ChEBI: A terminal acetylenic compound that is (17alpha)-17-ethynylestra-1(10),2,4-triene substituted by a methoxy group at position 3 and a hydroxy group at position 17.
A stirred solution of 120 parts of 3-methoxy-δ1,3,5-estratrien-17-one in 2,600 parts of anhydrous toluene and 4,300 parts of anhydrous ether is saturated with a slow stream of acetylene. In the course of 30 minutes there is added a solution of 120 parts of potassium tert-amylate in 2,800 parts of anhydrous tert-pentanol. The passage of acetylene and stirring are continued for an additional 5 hours after which the reaction mixture is washed 5 times with 3,000-part portions of saturated ammonium chloride solution and then with water. It is then dried over anhydrous sodium sulfate and concentrated to dryness under vacuum. The residue is recrystallized from methanol. The 3- methoxy-17-ethynyl-δ1,3,5 estratrien-17-ol thus obtained melts at about 143° to 146°C. A further recrystallization from acetone yields crystals melting at about 150° to 151°C.
Confirmed carcinogen
with experimental. neoplastigenic,
tumorigenic, and teratogenic data.
Moderately toxic by subcutaneous route.
Human reproductive effects by ingestion:
changes in ovaries and fallopian tubes,
ferthty effects. Experimental reproductive
effects. Mutation data reported. An FDA
proprietary drug. A steroid used in oral
contraceptives. When heated to
decomposition it emits acrid smoke and
irritating fumes.