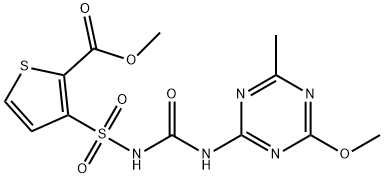
Postemergence herbicide used to control wild garlic and many broad-leaved weeds
in barley and spring wheat.
ChEBI: A methyl ester resulting from the formal condensation of the carboxy group of thifensulfuron with methanol. It is used as a post-emergence herbicide for the control of grass and broad-leaved weeds.
Herbicide: A herbicide for postemergence broadleaf weed control
in crops for food such as soybeans and cotton. Not
listed for use in EU countries.
ALLY®; BASIS® (rimsulfuron + thifensulfuron
methyl); CANVAS® (thifensulfuron methyl + tribenuron
methyl + metsulfuron-methyl); DPX-M6316®;
EXPRESS®; HARMONY® Extra (thifensulfuron
methyl + tribenuron methyl); INM-6316®; PINNACLE®;
PROSPECT®; RELIANCE® SYNCHRONY®, (chlorimuron-
ethyl + thifensulfuron methyl)
Chemical/Physical. May hydrolyze in aqueous solutions forming methyl alcohol and
3-(((((4-methoxy-6-methyl-1,3,5-triazin-2-yl)amino)carbonyl)amino)sulfonyl)-2-
thiophenecarboxylic acid.
The hydrolytic degradation of thifensulfuron methyl is
pH dependent and, in alkaline condition, specifically
yields the corresponding free acid. Primary
degradation cleaves the sulfonylurea moiety to give
two typical hydrolyzed products, sulfonamide and
aminotriazine analogs, derived from thifensulfuron
methyl in acidic and neutral conditions. Hydrolysis of
the ester linkage proceeds in mammals, plants, soils, and also chemical hydrolysis, and opening of the
triazine ring occurs to yield the acetyltriuret analog
identified. On the other hand, by hydrolysis, O-
demethylated thifensulfuron methyl undergoes opening
of the triazine ring to give the corresponding
acetyltriuret analog. Under photolytic conditions,
methyl 3-(4-methoxy-6-methyl-1,3,5-triazin-2-
yl)aminothiophene-2-carboxylate is identified.