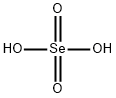
colourless or white crystals
Selenic acid is used as an oxidizing agent. Its derivative sodium selenate is used in the preparation of glass and animal feeds. It acts as an analytical reagent and also used for the preparation of other selenium salts such as gold(III) selenate. Further, it reacts with fluorosulfuric acid to get selenoyl fluoride.
ChEBI: Selenic acid is a selenium oxoacid. It is a conjugate acid of a hydrogenselenate.
A white crystalline solid. Very corrosive to skin, eyes and mucous membranes. Corrosive to metal. Toxic by skin absorption and by ingestion.
Very hygroscopic. Soluble in water.
Selenic acid, LIQUID reacts exothermically with chemical bases (for example: amines and inorganic hydroxides) to form salts. The reactions can generate dangerously large amounts of heat in small spaces. A good oxidizing agent. May oxidize organic matter such as wood, cotton, fiberboard, etc.. Reacts with active metals, including iron and aluminum, and also less active metals, to dissolve the metal and liberate hydrogen and/or toxic gases. Can initiate polymerization in certain classes of organic compounds. Reacts with cyanide salts and compounds to release gaseous hydrogen cyanide. Flammable and/or toxic gases are also often generated with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and weak or strong reducing agents. Additional gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and even carbonates: the carbon dioxide gas from the last is nontoxic but the heat and spattering from the reaction can be troublesome. May often catalyze (increase the rate) of chemical reactions.
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Selenium compounds are poisons. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of Se. See also SELENIUM COMPOUNDS,