Diflubenzuron is an insecticide of the benzoylurea class.It is used in forest management and on field crops to selectively control insect pests, particularly forest tent caterpillar moths, boll weevils, gypsy moths, and other types of moths.It is a widely used larvicide in India for control of mosquito larvae by public health authorities. Diflubenzuron is approved by the WHO Pesticide Evaluation Scheme.
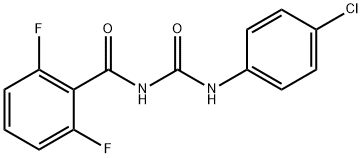
Diflubenzuron is produced by the reaction of 2,6-difluorobenzamide
with 4-chlorophenylisocyanate. Diflubenzuron
was first registered as a pesticide in the United States in 1979.
Environmental Protection Agency issued a Registration Standard
for diflubenzuron in September 1985 (PB86-176500). In
1991, a Data Call-In was made to require additional residue
chemistry and ecological effects data. The current Reregistration
Eligibility Decision Document was issued in August 1997,
reflecting analysis of the new data.
Diflubenzuron is a benzoylphenylurea insecticide that inhibits chitin synthesis in insects with an IC50 value of 0.611 nM for 14C-labeled N-acetyl-D-glucosamine incorporation in the cockroach. Specifically, it inhibits chitin synthetase at the egg and larval stages, leading to an inability to exit the egg or exocuticle, respectively. Diflubenzuron is genotoxic and mutagenic in mice at doses of 0.3, 1, and 3 mg/kg. Formulations containing diflubenzuron are used primarily in agricultural applications but are also used to control insects in livestock production.
Diflubenzuron is a benzoylurea-based pesticide belonging to the benzamide class. Diflubenzuron is a chitin synthesis inhibitor. Diflubenzuron is used in both agriculture and forest management to selec
tively control insect pests, particularly moths and weevils.
nsystemic insecticide used to control leaf-eating larvae and leaf miners in forestry, woody ornamentals and fruit trees.
Diflubenzuron is used for the control of a wide range of leaf-eating
insects in forestry, woody ornamentals and fruit. It controls major pests
on cotton, soyabean, citrus and tea and it also controls larvae of flies,
mosquitoes and locusts. Diflubenzuron is also used as an ectoparasiticide.
An inhibitor of TCDD-induced CYP1a1 expression in HepG2 cells.
ChEBI: A benzoylurea insecticide that is urea in which a hydrogen attached to one of the nitrogens is replaced by a 4-chlorophenyl group, and a hydrogen attached to the other nitrogen is replaced bgy a 2,6-difluorobenzoyl group.
Colorless to yellow crystals. Used as a selective insecticide.
Hydrolyzed in alkaline solution above pH 9.0.
Insecticide, Larvicide: Diflubenzuron is used primarily on citrus, cattle
feed, cotton, forestry, mushrooms, ornamentals, pastures,
soybeans, standing water, sewage systems, and wide-area
general outdoor treatment sites. The insecticide behaves as
a chitin inhibitor to inhibit the growth of many leaf-eating
larvae, mosquito larvae, aquatic midges, rust mite, boll weevil, and house-black-, and stable-flies. Diflubenzuron was
first registered in the United States in 1979 for use as an
insecticide.
ADEPT®; ASTONEX®; DIMILIN®;
DIMILIN® FLO; DIMILIN® WG-80; DU-112307®;
DUPHAR® PH 60-40; ODC-45®; DIFLURON®;
DU 112307®; LARGON®; LARVAKIL®;
MICROMITE®; OMS 1804®; PDD 60401®; PH 60-
40®; PHILIPS-DUPHAR® PH 60-40; TH 60-40®;
THOMPSON-HAYWARD® 6040; VIGILANTE®
Moderately toxic by
skin contact. Mildly toxic by ingestion.
Mutation data reported. When heated to
decomposition it emits very toxic fumes of
Cl-, F-, and NOx.
Soil. The half-life in soil is <1 week (Hartley and Kidd, 1987). Di?ubenzuron degrades more rapidly in neutral or basic conditions but more slowly under acidic conditions (pH <6) (Ivie et al., 1980).
Chemical/Physical. Hydrolyzes in water to 4-chlorophenylurea (Verschueren, 1983).
Diflubenzuron was the first active substance commercialised as a
benzoylurea insect growth regulator and there is extensive published
information on its degradation and metabolism. Detailed studies of the
degradation in soils have shown that cleavage of the urea linkage is
the major process. This also occurs in plants, insects and mammals but
the formation of products in which diflubenzuron is hydroxylated in
both rings is also an important metabolic process.
Diflubenzuron was shown to be stable to hydrolysis in aqueous solution
at acidic pH (DT50> 56 days at pH 4) but was readily hydrolysed at pH 10
(DT50 <3 days). In distilled water the DTa was 7 days (Ivie et al., 1980).
The major degradation products isolated were 4-chlorophenylurea (2)
and 2,6-difluorobenzoic acid (3). An additional minor product was 2,6-
difluorobenzamide (4). The hydrolysis products are shown in Scheme 1.
Additional products were formed under extreme conditions (121 °C
under pressure).
Aqueous solutions of diflubenzuron are reported to be unstable to light
but the solid is stable in sunlight (PM).
Diflubenzuron is an odorless, white, crystalline solid with
a melting point of 230–232 �C. It is almost insoluble in water (0.2 mg l�1) and poorly soluble in apolar organic solvents.It is almost nonvolatile. It is relatively stable in
acidic and neutral media but hydrolyses under alkaline
conditions.
Diflubenzuron is difficult to be degraded in sterilized water
under neutral or acidic conditions. However, it is degraded
rapidly under field conditions. Application of diflubenzuron to
water resulted rapid partition to sediment; the parent
compound and 4-chlorophenylurea (CPU) may persist on
sediment for more than 30 days.
The rate of degradation of diflubenzuron in soil is strongly
dependent on the particle size. For larger particles
(10 microns), the half-life is 8–16 weeks and for smaller
particles (2 microns), it is 0.5–1 week. Almost all of the parent
compound breaks down to form 2,6-difluorobenzoic acid
(DFBA) and CPU. A very minor amount forms 4-chloroaniline
(PCA) which rapidly binds to the soil. Under field conditions,
diflubenzuron has very low mobility.
Very little diflubenzuron is absorbed, metabolized, or
translocated in plants. It also is not readily taken up from
treated soil.
Diflubenzuron has very low vapor pressure (<2×10-7 Pa
at 25°C) and its atmospheric half-life is only several hours.
Therefore, it is not expected that diflubenzuron will be present
in air for extended periods and the long-range transport and
redeposition of diflubenzuron is expected to be negligible.