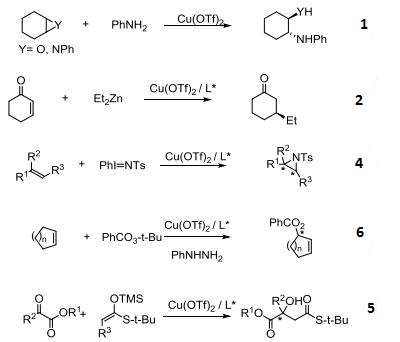
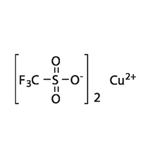- Ring-Opening of epoxides and aziridines.
- Asymmetric conjugate addition of organozinc reagents to α,β-unsaturated ketones.
- Electrophilic addition of olefins.
- Asymmetric aziridination of olefins.
- Asymmetric cycloadditions and aldol condensations.
- Asymmetric Kharasch oxidation.
- Asymmetric Michael addition of enamides.
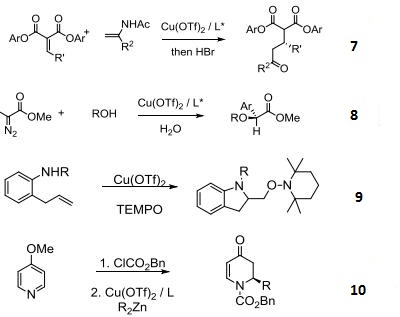
- Asymmetric O-H or O-R insertion reactions.
- Enantioselective intramolecular aminooxygenation of alkenes.
- Enantioselective addition of dialkylzinc reagents to N-acylpyridinium salts.
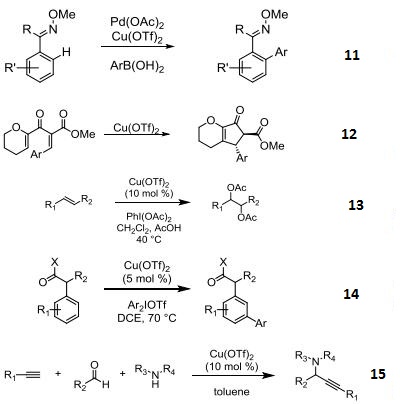
- Pd-catalyzed C-H functionalizations of oximes with arylboronic acids.
- Used as a Lewis acid in the Nazarov cyclization.
- Catalyst in the diacetoxylation olefins.
- Catalyst in the meta-selective direct arylation of α-aryl carbonyl compounds.
- Catalyst in the three-component coupling of amines, aldehydes, and alkynes.
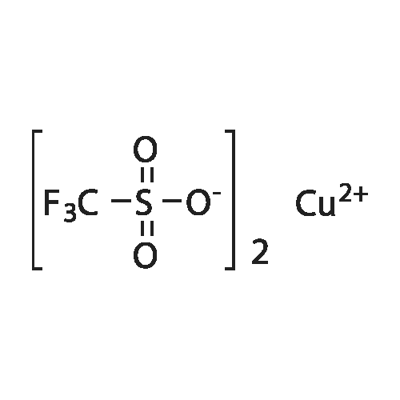
white to slightly blue or light grey cryst. powder
Copper(II) trifluoromethanesulfonate is a mild lewis acid. It is used as catalyst which promotes dehydration of alcohols and diols to alkenes at ambient temperatures. It is widely used to generate carbenoid species from ?-diazo esters and ketones, via in situ reduction to the Cu(I) species. It is also promotes the reaction between diazo esters and imines to give aziridines. It catalyzes syn-selective aldol condensation of (Z)-silyl enol ethers with aldehydes, Friedel-Crafts alkylation, acylation reactions of aromatics and addition of trimethylsilyl cyanide to carbonyl compounds.
Dissolve it in MeCN, add dry Et2O until cloudy and cool at -20o in a freezer. The light blue precipitate is collected and dried in a vacuum oven at 130o/20mm for 8hours. It has 737nm (� 22.4 max M1cm -1) in AcOH. [Salomon & Kochi J Am Chem Soc 95 330 1973]. It has also been dried in a vessel at 0.1Torr by heating with a Fischer burner [Andrist et al. J Org Chem 43 3422 1978]. It has been dried at 110-120o/5mm for 1hour before use and forms a *benzene complex which should be handled in a dry box because it is air sensitive [Kobayashi et al. Chem Pharm Bull Jpn 28 262 1980, Salomon & Kochi J Am Chem Soc 95 330 1973]. [Beilstein 3 IV 34.]