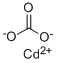Cadmium carbonate is precipitated by adding excess ammonium carbonate to a solution of cadmium chloride:
CdCl2 + (NH4)2CO3 → CdCO3 + 2NH4Cl
The precipitate is filtered and dried at 100°C. If an alkali metal carbonate is used instead of ammonium carbonate, a hydrated basic carbonate is obtained which upon heating with ammonium chloride at 150°C in the absence of air produces anhydrous carbonate.
Cadmium carbonate also may be obtained by slow absorption of cadmium oxide with carbon dioxide.
White powdery solid; density 4.258 g/cm3; decomposes on heating below 500°C; insoluble in water and liquid ammonia; soluble in acid (with reaction).
White powdery solid; density 4.258 g/cm3; decomposes on heating below 500°C; insoluble in water and liquid ammonia; soluble in acid (with reaction).
Cadmium carbonate occurs in nature as the mineral otavite. The commer_x0002_cial applications of this compound are limited. It is used as a catalyst in organic synthesis and as a starting material to prepare other cadmium salts.
Cadmium carbonate is the raw material for the production of pigments for ceramic and frits and for production of cadmium salt. Used as reagents. It is used for intermediates of manufacturing polyester, glass pigment flux, organic catalysts, plastic plasticizer, stabilizer.
Confirmed human
carcinogen. Poison by ingestion. Mutation
data reported. When heated to
decomposition it emits toxic fumes of
cadmium.