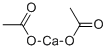Calcium acetate, also known as acetate of lime or vinegar salts, is the calcium salt of acetic acid. It is an odorless powder. Calcium has an important role in the nutrition of humans and animals. Hormonal mechanisms control absorption of dietary calcium (including added calcium salts) allowing adaptation to a range of calcium intakes while maintaining a relatively constant blood calcium concentration of about 10 mg/100 mL. Major functions of calcium inside the body include the formation and maintenance of bones and teeth, the physiology of muscle contraction, the cell membrane integrity, the activity of several enzymes that have specific requirement for it, the coagulation of blood, and the regulation of acid-base balance.*
Calcium acetate [Ca(CH3COO)2.H2O] is used as a food additive and a mordant to fix dyes in the textile industry. It is used as an alkali (base) in the manufacture of soaps, to improve some lubricants, and as an antimold to preserve baked goods for a longer shelf life.
ADI is not subject to restrictive regulations (FAO/WHO, 2001).
GRAS (FDA, §181.29, §182.6197, § 184.1185, 2000).
LD50: 52 mg/kg (mouse, subcutaneous).
GB 14880 a 94: cereals and their products, beverages 8~16g/kg.
GB 2760-2001: vinegar, 6~8g/kg (in terms of Ca).
FAO/WHO (1984): Edible caseinate, GMP.
FDA, § 184.1185 (2000): baked goods, gelatin, pudding, fillings, 0.2%; sweet sauce, top materials and poured, 0.15%;
EEC can be used for packaging cheese powder, quick-setting jelly powder.
FEMA (mg/kg): soft drinks 200; baked goods 500.
It is obtained by the reaction between calcium carbonate and acetic acid. Preparation method of anhydrous calcium acetate: the refined powder of calcium carbonate is added to the water, stirred into a suspension; added separately of a small amount of glacial acetic acid. After completion of the reaction, the filtrate was concentrated in a water bath and a white solid, anhydrous calcium acetate, was precipitated from the viscous filtrate.
It is obtained by the neutralization between coke acid (wood acetic acid) and calcium hydroxide, followed by the evaporation of the filtrate and recrystallization.
It is obtained by the reaction between the reaction between acetic acid and calcium hydroxide or calcium carbonate. Filter, concentrate and cool to obtain the dihydrate (colorless crystal), heated to 84 °C in a water salt (colorless crystal), heated to 100 °C to obtain the anhydrous salt.
Shellfish can be taken as raw materials, washed, crushed and dried for 1 h, subjecting to barbecue for 2 hour at 900~l000 ℃, then being added water to make a 1.3~1.5mol/L lime milk. And then neutralized with acetic acid to clarify, filter with the filtrate concentrated, and finally dried at 120~140 °C to get the final product with a yield of 91.28%.
To a 20% acetic acid solution, add calcium carbonate powder to until there is no longer any CO2 gas escaping, then add a small amount of calcium carbonate, heated 80 ° C for reaction of 2-3h. The filtrate was heated and concentrated with water bath while adding a small amount of 80% acetic acid at the same time to precipitate the calcium acetate monohydrate (what precipitated after the cooling of the solution is dihydrate), and finally dried at 60~70 ℃to derive the products.
Hazards & Safety Information
Category: Toxic substances
Toxicity classification: highly toxic
Acute toxicity: intravenous-mouse LD50: 52 mg/kg; celiac-mouse LD50: 75 mg/kg
Flammability and Hazardous characteristics: Thermal decomposition releases Pungent irritation Smoke
Storage and transportation characteristics:Treasury: ventilated, low temperature drying; store it separately from food raw materials
Fire extinguishing agent: water, dry powder, foam, carbon dioxide
Calcium acetate is a chemical compound which is calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous form is very hygroscopic; therefore the monohydrate (Ca(CH3COO)2?H2O) is the common form.
Calcium acetate occurs as a white or almost white, odorless or
almost odorless, hygroscopic powder.
Calcium Acetate is the calcium salt of acetic acid which functions
as a sequestrant and mold control agent. it contains approximately
25% calcium. it is a white odorless powder which is readily soluble
in water with a solubility of approximately 37 g in 100 g water at
0°c. its solubility decreases with increasing temperature, with a sol-
ubility of approximately 29 g in 100 g of water at 100°c.
Calcium Acetate is a salt of acetic acid (A167640), a common chemical reagent used in a multitude of organic reactions. It is the primary constituent of vinegar, contributing to its distinct taste and odor. It is used in the synthesis of dye-sensitized solar cells.
Calcium Acetate is the salt of acetic acid which is used as a preservative and sequestrant.
Calcium acetate can be prepared by soaking calcium carbonate (found in eggshells, or in common carbonate rocks such as lime stone or marble) in vinegar:
CaCO3 + 2CH3COOH → Ca(CH3COO)2 + H2O + CO2
Since both reagents would have been available pre-historically, the chemical would have been observable as crystals then.
Produced by calcium hydroxide neutralization of acetic acid.
ChEBI: The calcium salt of acetic acid. It is used, commonly as a hydrate, to treat hyperphosphataemia (excess phosphate in the blood) in patients with kidney disease: the calcium ion combines with dietary phosphate to form (insoluble) calcium phosphate, which is
excreted in the faeces.
Calcium Acetate belongs to the group of calcium salts, widely used as phosphorus binders in patients with chronic renal failure.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical Applications
Calcium acetate is used as a preservative in oral and topical
formulations.
Therapeutically, parenteral calcium acetate acts as a source of
calcium ions for hypocalcemia or electrolyte balance. Oral
calcium acetate is used as a complexing agent for hyperphosphatemia
in dialysis patients. Calcium acetate is also used in the
food industry as a stabilizer, buffer and sequestrant.
Poison by intravenous
and intraperitoneal routes. Mutation data
reported. See also CALCIUM
COMPOUNDS. When heated to
decomposition it emits acrid smoke and
fumes.
Calcium acetate is used in oral and topical formulations. The pure
form of calcium acetate is toxic by IP and IV routes.
LD50 (mouse, IP): 0.075 g/kg
LD50 (mouse, IV): 0.052 g/kg
LD50 (rat, oral): 4.28 g/kg
Veterinary Drugs and Treatments
Calcium acetate can be used for oral administration to treat hyperphosphatemia
in patients with chronic renal failure. Secondary to its
phosphorus binding efficiency and lower concentration of elemental
calcium, calcium acetate is considered the most effective and having
the lowest potential for causing hypercalcemia of the calcium-based
phosphorus-binding agents. When compared to calcium carbonate,
calcium acetate binds approximately twice as much phosphorus per gram of elemental calcium administered. Unlike calcium citrate, calcium
acetate does not promote aluminum absorption.
Potentially hazardous interactions with other drugs
Can impair absorption of some drugs, e.g. iron,
ciprofloxacin.
The residual acetate will be metabolised through
bicarbonate, which will be further excreted via normal
metabolic routes.
Any unbound calcium not involved in the binding
of phosphate will be variable and may be absorbed.
Calcium is absorbed mainly from the small intestine by
active transport and passive diffusion. About one-third
of ingested calcium is absorbed although this can vary
depending upon dietary factors and the state of the small
intestine. 1,25-Dihydroxycholecalciferol (calcitriol), a
metabolite of vitamin D, enhances the active phase of
absorption.
Excess calcium is mainly excreted renally. Unabsorbed
calcium is eliminated in the faeces, together with that
secreted in the bile and pancreatic juice. Minor amounts
are lost in the sweat, skin, hair, and nails.
Calcium acetate is stable although very hygroscopic, and so the
monohydrate is the common form. It decomposes on heating (above
1608℃) to form calcium carbonate and acetone.
Store in well-closed airtight containers.
Recrystallise it from water (3mL/g) by partial evaporation in a desiccator. [Beilstein 2 IV 113.]
Calcium acetate is incompatible with strong oxidizing agents and
moisture.
GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (oral suspensions
and tablets; topical emulsions, lotions, and creams). Included
in nonparenteral medicines (oral tablets) licensed in the UK.