The in vitro perfusion of the human placental lobule with 7-ethoxycoumarin gives 7-hydroxycoumarin, and perfusion with androstenedione gives the conversion products, estrone, estradiol, and testosterone. The human placenta has only a limited capacity for the metabolism of xenobiotics.
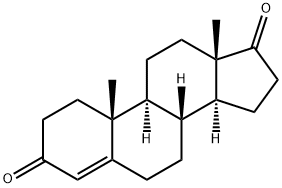
Androstenedione (CRM) (Item No. ISO60161) is a certified reference material categorized as an anabolic androgenic steroid. Androstenedione is a precursor of testosterone (Item Nos. ISO60154 | ISO00154 | 15645) and estrone . Androstenedione is regulated as a Schedule III compound in the United States. Androstenedione (CRM) (Item No. ISO60161) is provided as a DEA exempt preparation. This product is intended for research and forensic applications.
Testosterone precursor and metabolite with androgenic activity.
Controlled substance (anabolic sterid)
ChEBI: A 3-oxo Delta4-steroid that is androst-4-ene substituted by oxo groups at positions 3 and 17. It is a steroid hormone synthesized in the adrenal glands and gonads.
An experimental teratogen. Otherexperimental reproductive effects. Questionablecarcinogen with experimental tumorigenic data. Whenheated to decomposition it emits acrid smoke andirritating fumes.
Crystallise the dione from hexane. It is soluble in *C6H6, CHCl3 and EtOH. It has max at 239nm. It is a precursor of estrone or testosterone and has androgenic activity. [Ruzicka & Wettstein Helv Chim Acta 18 980 1935, Beilstein 7 III 3636, 7 IV 2381.]